기사본문
Yooyoung Pharmaceutical, 'IL13Rα2-CAR-T' development for Glioblastoma
입력 2018-05-17 09:22 수정 2018-05-18 14:19
by Euna Lee
YooYoung Pharma, a specialized drug development company, challenges ‘glioblastoma’, not blood cancer, with a ‘CAR-T medicine’. Since 2007, YooYoung Pharma has been engaged in research and development of biopharmaceuticals through open innovation. This year is the fourth year for them to conduct CAR-T study.
YooYoung Pharma’s executive vice president Dr. Jung Ju Kim said, “CAR-T is currently in the non-clinical phase, and aims to submit an investigational new drug (IND) in Korea for patients with relapsed or refractory glioblastoma early next year.” In addition, YooYoung Pharmaceutical’s new drug pipeline includes 10 INDs, including HGF antibodies, NASH drugs, and CAR-T. The HGF antibody is at the end of phase 1 clinical trial.

▲Dr. Jung Ju Kim, Executive vice president of YooYoung pharmaceutical
YooYoung has recorded sales of 91.8 billion won last year and plans to devote 16 % of its target sales to R&D this year. , They invested (6.9 and 9.8) % in 2016 and 2017 in R&D, and this year, further significantly increased the proportion of R&D. Dr. Kim said confidently, “This year, we will stick -with our standard core values and make aggressive investments. We will make a new leap forward in YooYoung pharmaceuticals with developing innovative new drugs that include CAR-T.
◇ IT, IV, and ICV administration in animal models was confirmed effective.. IND targets at the beginning of next year
‘YYB-103’, one of YooYoung’s pipelines, is CAR-T cell targeting IL13Rα2. IL13Rα2 (interleukin-13 receptor α-chain variant 2) is expressed in approximately (50–80) % of the glioblastoma surface, and rarely appears in normal tissue.
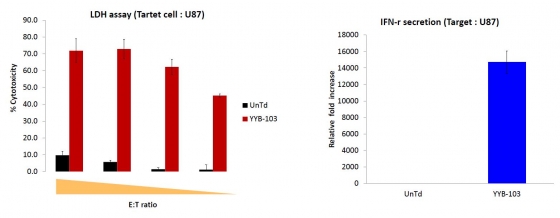
▲'YYB-103' 'YYB-103' showed excellent cytotoxic effect on brain cancer cell line (U87) and increased IFN-γ secretion. (Reference: provided by YooYoung pharmaceutical)
Dr. Kim emphasized, “The City of Hope study team, conducting CART-IL13Rα2 clinical trial, administered it directly to brain tissue. Apart from the therapeutic effect, intracranial administration is dangerous in itself, and has serious side effects. YooYoung Pharmaceutical was effective in all three dosing regimens in animal models: intra-tumoral (IT), intracerebroventricular (ICV), and intravenous (IV) administration. If YYB-103 that will be administered with IV shows efficacy in clinical trials ,it will have enough global competitiveness.”
“YYB-103 modifies IL13 to improve selectivity for IL13Rα2 and reduce toxicity. Flexibility was also increased by adding a linker between the IL13 portion and the hinge region. Finally, the target specificity, CAR-T expression rate, and structural stability were both increased”, he explained.
Then what about the non-clinical outcome of YYB-103? In conclusion, YYB-103 migrated into the brain tumor through the BBB of the mice, and CAR-T cells were actually working. Let’s start with the in vitro data. “The cytotoxic effect of YYB-103 is higher compared to the in vitro data seen in other team CAR-T cells, and IFN-γ secretion is also very high in U87 cells”, said Dr. Kim.
In vivo efficacy was also confirmed in glioblastoma animal models. Notably, in the orthotropic xenograft mouse model that planted a tumor cell line into the brain, YYB-103 was directly injected intra-tumoral (IT), intracerebroventricular (ICV), and intravenous (IV), and all showed anticancer effect.
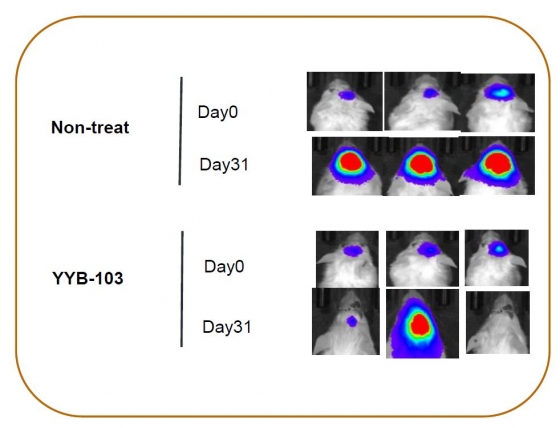
▲In the glioblastoma animal model, the survival period of 'YYB-103' alone group was extended by 20% compared to the control group. (Reference: provided by YooYoung pharmaceutical)
“YYB-103 was directly administered intra-tumoral(IT), and the tumor response was observed within 7 days, showing excellent efficacy. In addition, the survival time was increased in a dose-dependent manner”, he added. The survival rate was 12.5 % for the intermediate dose of YYB-103, and 37.5 % for the high dose group, whereas the entire control group died after 5 months of transplantation of U87 tumor cells in the mouse. The mean survival time increased by (32 and 57) %, respectively, compared to control group.
Intravenous (IV) administration slowed tumor growth, and prolonged survival. Dr. Kim said, “Intravenous administration of YYB-103 monotherapy resulted in a 20 % prolongation of survival compared to the control group. Intravenous administration of CART-EGFRvIII showed more than double the survival extension, compared to (8 to 9) days of survival extension from the Duke University research team.”
Finally, intracerebroventricular (ICV) administration was also effective. Synergistic effects were observed here by co-administration with temozolomide (TMZ). “The non-treat group, which did not receive any treatment, had brain tumors activated on day 36, and only some of the anti-cancer effects were seen in the TMZ-mono treated group”, Dr. Kim said. “On the other hand, when YYB-103 was co-administered after pretreatment with TMZ, the cancer completely disappeared”, he explained.
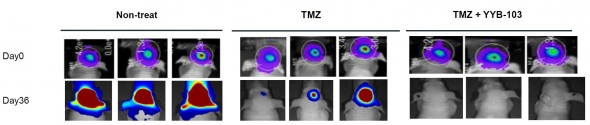
▲In vivo efficacy results in intra-cerebroventricular (ICV) administration of 'YYB-103' (Reference: provided by YooYoung pharmaceutical)
Dr. Kim said, “As a result, we observed the effects of YYB-103 on all IT, ICV, and IV administration in animal experiments. It is a further challenge to be able to pass brain tumors through the BBB via IV. Currently, YYB-103 evaluates its efficacy in patient-derived xenograft (PDX) animal models. Clinical trials are planned for IV. The GMP facility is looking for several organizations that can cooperate, including the CMO.”
◇ Developed HGF antibody, NASH targeted new drug... … established a subsidiary ‘Autophagy science’
CAR-T cells ‘YYB-103’, anti-HGF antibodies ‘YYB-101’ and NASH drug candidates are representative innovative new drugs of YooYoung Pharmaceutical. The leading pipeline, YYB-101, is a humanized neutralizing antibody targeting hepatocyte growth factor (HGF), and is in the final phase of clinical phase 1.
“YYB-101 has demonstrated safety in clinical trials in colorectal, melanoma, and ovarian cancer patients”, said Dr. Kim. “Unlike competitive drugs, YYB-101 is designed to be a new structure, and it challenges the development of HGF inhibitors that have failed in global companies”. “We will plan Phase 2 by selecting the type of cancer that shows effective treatment in clinical phase 1”, he said.
Candidates for NASH drug are also expected. It has a mechanism to treat hepatitis by promoting the activity of autophagy. YooYoung Pharmaceutical has established its subsidiary ‘Autophagy science’ to concentrate on the development of new drugs for autophagy related disease. Dr. Kim said, “We will target NASH in the first place, and expand the indications due to fibrosis and degenerative brain disease, which are autophagy related diseases”.
“Currently, we are building various pipelines, including antibodies, CAR-T, and NASH, and plan to focus based on selection through spin-offs. In addition to the development of new drugs, we will also refine our specialty pharmaceuticals business to focus on the core. YooYoung Pharma plan to be listed in a few years, to secure funding for the expansion of ongoing new drug candidate research, clinical development, and advanced manufacturing facilities.”







