기사본문
Vision of 'GenoFocus', Microbiome Engineering for New Drug
입력 2018-05-24 15:53 수정 2019-01-24 18:24
by Euna Lee
GenoFocus, an industrial and pharmaceutical enzyme development company, has recently been making a full-fledged effort to develop a new concept of microbiome drugs potentiated with enzyme combination.
Eui-Joong Kim, CEO of GenoFocus, introduced the specialty of the company, saying, “We have developed a system to identify therapeutic candidates from GRAS (generally recognized as safe) bacterial strains, and deliver them to the gastrointestinal (GI) tract. This is Spore Biologics using Bacillus spores. Spores are used as vehicles to deliver enzyme or protein drugs displayed on the spore surface.
More interestingly, spores reach the intestine safely and then produce therapeutic proteins or enzymes in situ.” Being transient passengers of the GI tract, but adapted to carry out their entire sporulation cycle within this environment. Bacillus spores are to germinate and release therapeutic proteins into the GI tract.
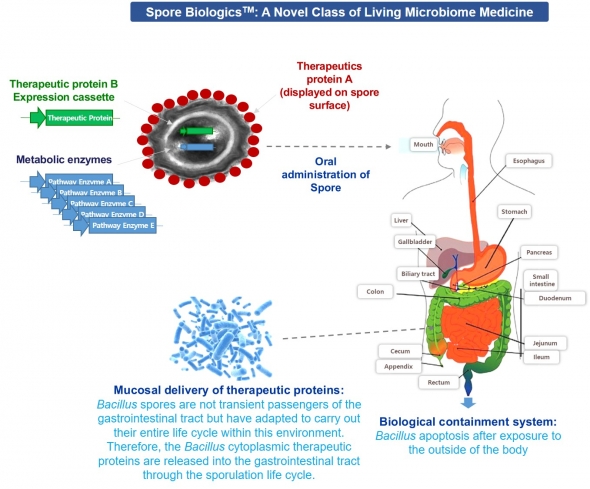
GenoFocus has been listed in the KOSDAQ market in 2015 as a new technology company, and has the platform technologies of ‘microbial display’ and ‘secretion expression’. Based on these core technologies, they have developed customized enzymes in various fields, such as food, medical and industrial enzymes. Among them, Lactase, Catalase, and Lipase are the representatives.... <계속>







