기사본문
Ubix Therapeutics develops PROTAC-based New Immuno-Anticancer Agent
입력 2018-11-12 09:40 수정 2019-01-28 21:30
by Euna Lee
Ubix Therapeutics, founded in June 2018, is currently promoting the commercialization of PROTAC for the first time among domestic companies, by introducing “Degraducer Technology”, the “PROTAC Technology” developed by the Korea Research Institute of Chemical Technology and the Korea Research Institute of Bioscience and Biotechnology through a Creative Convergence Research Project that is supported by the National Research Council of Science & Technology. In particular, the company is concentrating on the development of new drug targeting the epigenetics and new immune-checkpoint proteins. The company recently moved into the Seoul BioHub located in Hongreung, Seoul.
Seo Bo-gwang, the president of Ubix Therapeutics, said, “PROTAC is a strong technology for inhibitors that are capable of targeting the diseases that had been undruggable so far, and this technology is being spotlighted in the global market”, “The competitiveness of Ubix Therapeutics is based on the secured diversity of the PROTAC platform, which consists of ‘Target Ligand - Linker - E3 Ligase Binder’.”
President Seo graduated from the Dept. of Microbiology, Seoul National University, and his career has included work in JW Pharmaceuticals, Genexine, the In Vitro Diagnosis Division of SK Telecom, and Lifecore Partners; the experiences therein have became the foundation of this new startup in the Bio-pharmaceutical industry.
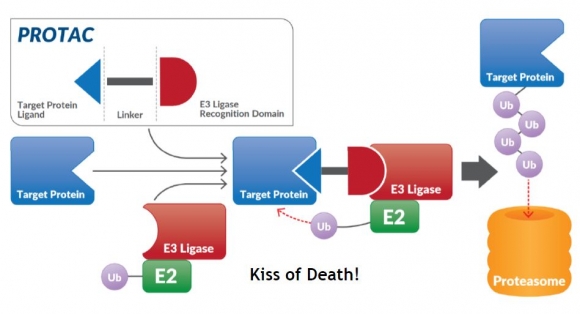
▲PROTAC 기술 모식도(유빅스테라퓨틱스 홈페이지)
◇ The ‘PROTAC’ technology enables Degradation of Disease causing Proteins – what is the strong point?... <계속>







