기사본문
Lunit received Class 3 Approval of AI breast cancer diagnostic medical device from MFDS
입력 2019-08-07 09:35 수정 2019-08-07 09:35
바이오스펙테이터 Sungmin Kim 기자

Lunit, on the July 31th, announced that their own breast cancer diagnostic aid device has been approved by MFDS as a class 3. This is the second license that the Lunit received after last year’s approval of the chest x-ray device.
The device ‘Lunit insight MMG’ received domestic sales license (product name: Lunit INSIGHT for Mammography) on last 29th by MFDS. Lunit Insight MMG was jointly developed by Lunit, Yonsei Severance Hospital, Seoul Asan Hospital, and Samsung Seoul Hospital.
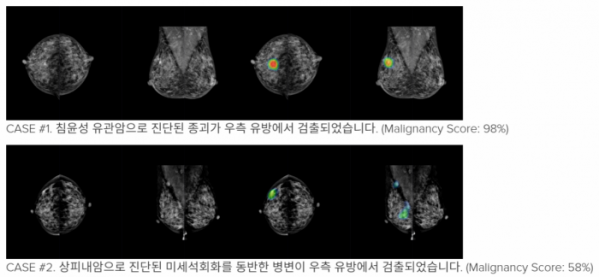
Lunit Insight MMG is screening assistant software for mammography imaging based on artificial intelligence technology, which helps physician diagnose quickly and accurately by marking suspicious areas and its degree of breast cancer.
Seo Bum Suk, CEO of Lunit, said "Breast cancer is specifically difficult in diagnosing and is one of the most commonly diagnosed women’s cancers in the world, accounting for 24% of all women’s cancers. Among the patients who were diagnosed suspicious for malignant tumor from the mammography, only 29% of the patients were confirmed to have cancer after histological examination. "
Seo added, "The product has been introduced to enhance the effectiveness of breast cancer screening and improve the reality of unnecessary testing due to low accuracy through artificial intelligence. With the use of Lunit Insight, the accuracy rate will be increased while rechecking rate will be reduced. "
According to the company, the diagnosis of breast cancer is more difficult for Korean and Asian women with dense breasts. Lunit Insight MMG uses only about 200,000 mammograms, including 50,000 cases of breast cancer, in deep learning to detect malignant tumors
"In order to overcome the high false positive rate which was a problem especially in the computer aided detection software, it was developed to distinguish between malignant and benign tumors with high accuracy," said Kim Hyo-Eun, managing director of Insight MMG. "The clinical trials to confirm the MFDS have proven (Lunit Insight MMG) significantly improves the accuracy of radiologists' readings."
Lunit designed to test the performance of the device through their website (https://insight.lunit.io). "Through online, anyone can test the performance of the Lunit Insight MMG," said Chang Minhong. "With the Lunit Insight MMG, we will not only detect breast cancer early, but also reduce the burden on patients due to unnecessary additional tests." he said.
In August last year, the Lunit Insight CXR, a device for chest X-ray analysis approved by FMDS, is being used by many hospitals and medical centers including Seoul National University Hospital.