기사본문
Abion Takes the Challenge to Combine ‘c-Met anticancer drug’ for Companion Diagnostics
입력 2019-08-27 09:15 수정 2019-08-27 09:15
by Joungmin Cho
Abion’s hepatocyte growth factor receptor (c-Met) target anticancer drug under development has been approved for phase 1/2a clinical trials in Korea following Australia. Abion will conduct global clinical trials of the anticancer drug at eight hospitals including Korea and Australia. Abion is targeting development of new drugs based on precision oncology. Precision oncology refers to anticancer treatment customized to individual patients based on biomarkers.
Chief Operating Offer (COO) and Vice President Choi Jun-Young said in a meeting with Biospectator, “Abion is developing its own customized companion diagnostics from an early stage of drug development. If we develop a companion diagnostic product for our new drug, we can effectively reduce production costs through accurate screening of patients during clinical trials, and it will benefit both patients and the company even after the new drug is released.”
◇ 'c-Met' target anticancer agent ABN401 “with chemical structure to solve kidney toxicity problem”
ABN401, the lead pipeline of Abion, is a small molecular compound with a mechanism that can selectively inhibit c-Met, a hepatocyte growth factor (HGF) receptor. c-Met is a transmembrane tyrosine kinase receptor, and if it binds with HGF, can cause phosphorylation of the tyrosine kinase domain inside the cell membrane, activating delivery of signals integral for cell growth and survival. It is mainly overexpressed in various types of solid cancers such as lung, stomach, and liver cancers. In addition to overexpression, amplification and mutation can be found, which are related to progression, metastasis, and prognosis of tumors.
Although c-Met was studied as a major anti-cancer treatment target with epidermal growth factor receptor (EGFR) for importance of its mechanism, it failed due to lack of safety and efficacy, and there has been no approved drug.
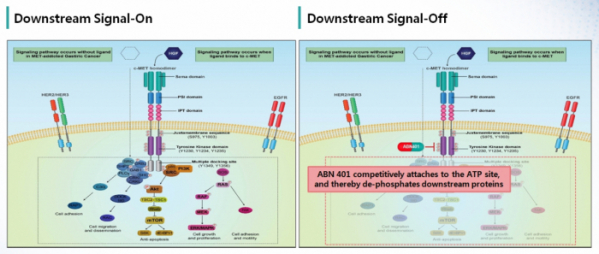
▲MOA of ABN401
Vice President Choi said, “The first-generation c-Met-targeted anticancer drug contained an aldehyde oxidase structure. It could produce insoluble substance through metabolism in the body, which builds up in the kidneys to cause a kidney toxicity problem.” He added that nephrotoxicity was the major cause of failure of the first-generation drug.
ABN401 can solve the toxicity problem by removing such structure from its initial structural design stage. Vice President Choi said, “When we conducted evaluation in the process introducing the technology from the Korea Research Institute of Chemical Technology, we found that it had a great advantage in terms of safety because there was no structure that could cause toxicity.”
The mechanism of action of ABN401 is also interesting. ABN401 acts competitively on the tyrosine kinase domain ATP site of c-Met to block protein phosphorylation of downstream signaling mechanism. It means that this mechanism can block activation of HGF-induced c-Met in tumors with occurrence of c-Met mutations, etc. and signal activation occurring independently from presence of HGF.
Abion evaluated the efficacy of ABN401 through a variety of cell and animal experiments. When ABN401 was applied to many tumor xenograft models and patient-derived xenograft (PDX) models, it showed high effect of inhibiting tumor growth in cases of carcinomas with high level of c-Met amplification or MET exon 14 deletion mutations.
After that, when ABN401 was applied to a non-small cell lung cancer tumor transplantation model in which amplification of c-Met occurred, it is found that it inhibits tumor growth in a dose-dependent manner. Additionally, when the combination of EGFR TKI and ABN401 was applied to a non-small cell lung cancer model in which c-Met and EGFR mutations occurred, it reduced the size of tumors by four times than the application of EGFR TKI only.
Vice President Choi said, “Among patients with non-small cell lung cancer patients who were treated with EGFR TKI, 20% of the patients had the occurrence of c-Met mutations. As ABN401 targets c-Met mutations, which is thought to be the cause of resistance against EGFR TKI, it is possible to additionally secure a patient group who develop resistance due to the occurrence of mutations after application of EGFR TKI.” Abion plans to develop ABN401 by selecting lung cancer as primary indication as it has the largest market.
◇ Simultaneous development of companion diagnostics from the beginning of development: “Development of cfNA and CTC-based liquid biopsy for companion diagnosis”
Abion has been developing companion diagnostics from the early stage of development of ABN401. Vice President Choi said, “As precision medicine becomes a trend, companion diagnostics for biomarkers are essential for the development of anticancer drugs. However, if one develops companion diagnostics after developing new drugs, it can cost a lot of money and the chances of success are very low.” This explains why new drug development companies should lead development of companion diagnostics.
By selecting c-Met gene amplification and exon 14 skipping as biomarkers based on mechanism of action of ABN401 and experimental data, Abion is developing a product that can detect and analyze them with liquid biopsy.
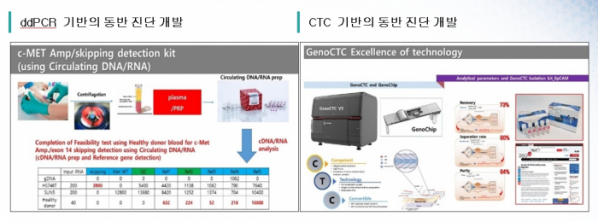
▲Companion diagnostic of ABN401
Vice president Choi said, “We are developing a companion diagnostic product that can detect biomarkers for cell free Nucleic acid (cfNA) through analysis with ddPCR method, etc.”
What is noteworthy in terms of companion diagnostics of Abion, the company is developing circulating tumor cell (CTC)-based diagnosis with cfNA. Vice President Choi said, “Those genetic mutations which are hardly detected in cfNA and tissues in the course of observing the progress after antic-cancer treatments can be confirmed by CTC-based analysis. Therefore, we are developing a CTC-based companion diagnostic because we think they will generate mutually complementary effect.”
◇ Phase 1/2a clinical trials in Australia and Korea' “We plan to expand it to the U.S. after confirming safety”
Abion has recently obtained approval for phase 1/2a clinical trial of ABN401 in Australia and Korea. To evaluate safety, pharmacokinetics, pharmacodynamics and anti-tumor activity of ABN401, this clinical trial is being conducted on approximately 190 subjects at eight institutions including four overseas institutions in Australia and four domestic institutions, such as the National Cancer Center, Seoul National University Hospital, Yonsei Severance Hospital, and Seoul Asan Hospital.
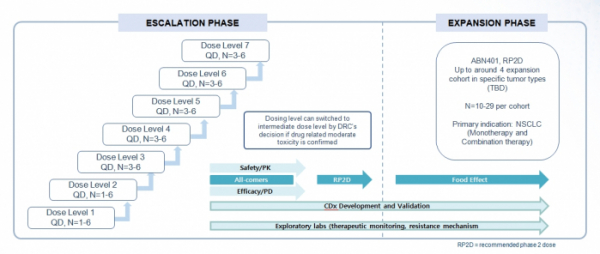
▲Clinical design of ABN401







