기사본문
Y-Biologics plans to enter a clinical trial on the first domestic PD-1 antibody.
입력 2020-02-03 09:46 수정 2020-02-03 09:46
by Yoonseok Suh
▲와이바이오로직스 박영우 대표(바이오스펙테이터)
The first domestic anti-PD-1 antibody candidate is about to enter the domestic Phase 1 clinical trial. Although 'IMC-001', an anti-PD-L1 antibody developed by IMMUNEONCIA, is now under the domestic Phase 1 clinical trial, 'YBL-006', an anti-PD-L1 antibody developed by Y-Biologics, has the same level of anti-cancer effect as 'Keytruda' by Merck and 'Opdivo' by BMS but uses a different binding site to PD-1.
Vice President Park Beom-Chan of Y-Biologics introduced the differentiated features of ALiCE bi-specific platform technology and the development status of new drugs at the "Daejeon Promising Bio Startup IR Conference for First Half-Year 2020" hosted by DailyPartners and held at Lotte City Hotel in Daejeon City.
Y-Biologics, established in 2007, is dedicated to R&D efforts using its self-developed human antibody library 'Ymax-ABL' under two strategies: the development of candidate substances to be administered in combination with anti-PD-1 antibody and the development of a T-cell bispecific ALiCE treatment drug. Y-Biologics plans to enter a domestic clinical trial for anti-PD-1 antibody and to go public on the KOSDAQ.
Y-Biologics's core pipeline YBL-006, an anti-PD-1 antibody, is an immune gateway inhibitor candidate which is aimed to enter Phase I clinical trial by 2020. The company will conduct the clinical trial by targeting non-small cell lung cancer and melanoma to show that YBL-006 exhibits higher antigen-binding capacity than 'Keytruda' and 'Opdivo,' and has similar anticancer effects to them. Vice President Park said, "YBL-006 is a novel antibody which uses a different epitope to bind to PD-1 compared to 'Keytruda' and 'Opdivo.'" In a cell model and an animal model, YBL-006 was found to reduce the size of cancer cells by a similar level to 'Keytruda' and 'Opdivo'. In addition, YBL-006 showed the drug toxicity similar to that of 'Keytruda' and 'Opdivo'.
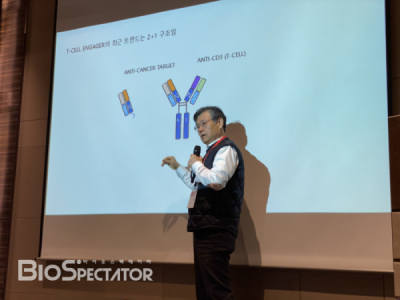
In addition, the company aims to complete the pre-clinical trial by 2021and facilitate global technology transfer during the pre-clinical trial phase of YBL-013 (ACE-05), a bispecific antibody using the ALiCE platform under development by Y-Biologics to target CD-3 and PD-1. The ALiCE bispecific antibody is a general Y-shaped IgG-type bispecific antibody with the '2+1' type binding capacity consisting of Fab, which targets two cancer antigens, and CD3 Fv, which targets T cell, and which exhibits anti-cancer effects and reduced toxicity. Y-Biologics confirmed that cancer cells in model animals administered with ACE-05 were removed more effectively than other drugs. Vice President Park added, "In the end, it all comes down to toxicity. Whether the candidate substance is evaluated as an effective drug depends on how it can minimize side effects by controlling toxicity."
Y-Biologics has concluded ten cases of joint development contracts and two cases of technology transfer contracts since 2016. The company aims to discover and develop self-developed candidate substances in a continuous way and to transfer technology based on its own human antibody library.
Vice President Park said, "Let me take Zymeworks for an example, which is a Canadian bispecific antibody developing company. Zymeworks has transferred bispecific antibody pipeline technologies to big pharmaceutical companies including Merck, Lilly, Celgene, and GSK from 2011 to 2018, and recorded the milestone payments of 116 million dollars from technology transfer in 2018. The remaining milestone payments are estimated at around 6.525 million dollars."