기사본문
TeraImmune, target autoimmune specific 'CAR-Treg development strategy'
입력 2020-02-27 13:44 수정 2020-02-27 13:44
바이오스펙테이터 Sungmin Kim 기자

The so-called 'CAR-Treg,' a therapeutic drug for regulatory T cells (Tregs) engineered with chimeric antigen receptor (CAR) targeting specific antigens that cause autoimmune diseases, is now moving from research phase to clinical phase. Tregs are immune cells that play a central role in balancing the immune system by regulating inflammatory responses. The concept of CAR-Treg started to appear in new drug development for autoimmune diseases a few years ago, but no case has entered a clinical trial yet. The biggest stumbling block is a production process that can obtain a sufficient number of Tregs.
As a solution to overcome this limitation, TeraImmune. Inc. has thrown down the gauntlet to the global Treg cell therapy field based on its Treg proliferation technology. The company name "TeraImmune" is the composite word of 'Tera (10^12)' and 'Immune,' which means a company that produces good cell therapy. TeraImmune was established in 2016 in Rockville, Maryland.
“The development of Trek cell therapies is an emerging field. In clinical development, the current challenge is to increase Tregs to a large scale enough to be administered to patients. TeraImmune has a stable Treg proliferation technology, as well as three patented core technologies. TeraImmune is expected to enter the first clinical trial as a disease-specific Treg cell therapy that targets a specific autoimmune disease. As there are only a limited number of competitors in the global CAR-Treg market, TeraImmune belongs to the top-tier group in this field. ”
CEO Kim Yong-Chan explained about the company's technology and differentiation approach in an interview with Biospectator. CEO Kim co-founded the company with Park Ji-hoon, a co-founder of CO2, whom he met at the National Institutes of Health (NIH) where he worked for over 15 years. The three core projects to develop disease-specific CAR-Treg cell therapies includes treatment-resistant Hemophilia A, multiple sclerosis drug, and CAR-Treg cell therapy, and B-cell lymphoma drug with a lower risk of recurrence.
Core Competitive Edge: ‘Stabil-T Technology’ and stable culturing of Foxp3+Helios+ Treg
Kim said, "There has yet been no established protocol for stable culturing of Tregs. TeraImmune's proliferation technology has the advantage of being able to obtain a high purity of Tregs with normal function."
TeraImmune's culturing technology has differentiation features in two aspects. First, it can be used to develop Foxp3+Helios+-expressing Tregs. CD4+CD25+Foxp3+ regulatory T cells can be divided into tTregs (natural Tregs; nTregs), which is differentiated from thymus, and pTregs, which is differentiated from peripheral tissues. Here, two markers (Foxp3+Helios+ Tregs) are considered as thymus-derived Tregs (tTregs).
Second, it has a culturing technology to obtain a high purity of Tregs in a stable way. CEO Kim said, "The existing technologies reduce the proliferation of other T cells than Tregs, while TeraImmune's proliferation technology uses the existing culturing technology but additionally treats with oligonucleotide to stabilize Treg mRNA."
To be specific, the existing culturing technology shuts down the proliferation of Tcon through treatment with rapamycin which can inhibit mTOR signaling pathway not used by Tregs. In addition, treatment with IL-2 is done to increase the number of Tregs. If it is applied to actual production process, it can obtain up to 60% purity of Tregs in three weeks. Here, TeraImmune has developed the "Stabil-T" platform, an oligodeoxynucleotide (ODN) treatment technology, as a method to stimulate and proliferate cells in order to obtain a high purity of Tregs. If this ODN treatment method is applied, it can obtain a high purity of Helios+Foxp3+ Tregs in four weeks. CEO Kim said, "Two genes, 'Foxp3 and Helios,' have a very short turn-over." ODN treatment is an approach to increase this turnover. IN 2012, Journal Blood released the study results on ODN-based proliferation technology (doi: 10.1182/blood-2011-09-377895).
The differentiation feature of Stabil-T platform which is emphasized by TeraImmune is "reproducibility." After testing the protocol by targeting 150 donors up to now, it was confirmed that a high purity (70-80%) of Helios+Foxp3+ Tregs. TeraImmune is now in the process of establishing a protocol for a cGMP-level production using its ODN treatment method.

Proliferated cells are used to produce CAR-Treg cell therapy. The production process of CAR-Treg is almost the same as that of CAR-T. If we look at the production process, blood sample is collected from a patient with autoimmune disease, and the wanted cells are isolated and extracted before CAR is delivered via viral vectors. After that, Stabil-t system is used to produce cells of specific dimensions, which is, in turn, administered to the patient. TeraImmune uses retrovirus to deliver receptors that bind target antigens to Treg cells. TeraImmune has obtained license for retroviral vectors from the National Cancer Institute (NCI) and signed an outsourcing supply contract for research and clinical trial purposes."
Overcome treatment-resistance in Hemophilia A...Expansion from FVIII TCR-T Treg to BAR Treg
Other key points in the development of Treg cell therapies are safety and potency. Those Treg cell therapies which have entered clinical trials up to now are all polyclonal adoptive cell therapies (ACT) that bind to multiple epitopes. However, there still remain the following problems: ▲concerns about adverse effects due to nonspecific immunosuppression mediated by polyclonal TCR stimulation, ▲a lack of Treg cells that detect antigens to generate immunosuppressive effect, and ▲a lack of potency. These problems indicate why it is difficult to move to commercialization stage.
Accordingly, the Treg cell therapy field is now moving toward developing CAR-Treg and TCR-Treg cell therapies that can target specific antigens which cause autoimmune diseases or alloimmune diseases in polyclones. This is expected to improve drug safety, potency, specificity and yield.
TeraImmune aims to treat autoimmune diseases using Tregs that target specific antigens. The first target is Hemophilia A patients resistant to treatments. Many Hemophilia A patients developed resistance after receiving FVIII treatment. Especially, 30% of severe patients developed resistance. The Immune Tolerance Induction (ITI) is used as a treatment for resistant patients, which induces immune tolerance by injecting a large amount of FVIII (25-200 IU/kg) to dilute neutralizing antibodies. Its problems is too frequent administration; about three to seven times per week.
To address these issues, TeraImmune takes up the challenge of solving the resistance problem in hemophilia A with Treg cell therapy. It comes up with a new strategy to inhibit Treg from recognizing other FVIIIs as antigens. TeraImmune plans to carry out two projects which target treatment-resistant Hemophilia A patients. First, it will conduct a clinical trial on FVIII TCR-Treg that targets T cell receptors (TCRs) first. Next, it will conduct another clinical trial on a universal FVIII BAR-Treg program. TeraImmune has already completed the patent registration for both programs.
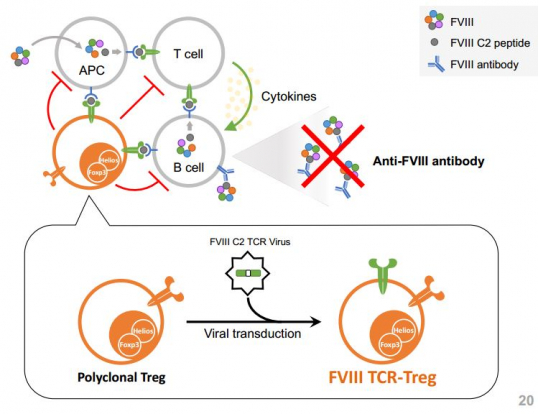
As soon as three types which target FVIII, such as TCR-Treg, CAR-Treg and BAR-Treg, are administered in a preclinical rat model of hemophilia, it was confirmed that it showed the inhibitory effect against the production of FVIII antibodies. In particular, B cell-targeting Antibody Receptor (BAR) is aimed to target B cells which produce resistant antibodies and is the optimized sequence for targeting BCR on the surface of plasma cells that secrete FVIII.
Strategically speaking, TeraImmune says that it will enter a clinical trial on autologous FVIII TCR-Treg first. The company plans to complete a pre-clinical test for IND filing by the second half of this year and expects to obtain clinical approval from the FDA in the first half of the next year. It has already formed a production partnership. TeraImmune will develop a production process for FVIII TCR-Treg with funds provided by the Partnership for Access to Clinical Trials (PACT) sponsored by the National Institutes of Health (NIH), and plans to produce clinical samples at Moffitt Cancer Center, a cGMP manufacturing agency hosted by the PACT.







