기사본문
RevoSketch, Targeting ‘Early Diagnosis’ of Intractable Diseases with ‘Digital PCR’
입력 2021-06-17 15:44 수정 2021-06-17 15:44
by Shinyoung Roh

“I think the diagnostic market will consider highly sensitive ‘digital PCR’ as the most important technology in the future. When the liquid biopsy market is opened through this high-sensitivity molecular diagnostic technology, a variety of diseases can be diagnosed using not only blood but also other human samples. The diagnostic market has the potential of explosive growth.”
RevoSketch CEO Seong-woon Lee presented RevoSketch's digital PCR device and technology at the 'Daily Partners D'LABs Demo Day' held at COEX Center in Gangnam-gu, Seoul on the 13th. RevoSketch, a company developing bioanalytical and diagnostic devices, was founded by CEO Lee in 2017. In February 2019, it attracted 2 billion won through Series A1.
CEO Lee said that RevoSketch is building a business model through cooperation with diagnosis-related companies based on the company's PCR devices and technology. Currently, RevoSketch is undergoing the process of discovering 20 types or more of early cancer biomarkers through a partnership with Bioneer. He explained that the discovered biomarkers had the potential to be developed for an early cancer screening service and such a blood test item could be included in the health examination in the future. In addition, RevoSketch is developing early diagnosis technology for Alzheimer through a partnership with Biorchestra, and is developing diagnostic technology for female diseases and infectious diseases such as COVID-19 with Seasun Biomaterials.
What is the difference between ‘digital PCR’ and conventional PCR? The biggest difference is 'sensitivity'.
PCR has been widely used in the field of molecular diagnosis because it can amplify and detect genes contained in the sample through amplification. However, using general PCR, it is difficult to diagnose a disease accurately or a disease at the early stage due to its low sensitivity. For example, when PCR is used to diagnose cancer, detection with general PCR is possible only in patients with cancer at the advanced stage 3 or late stage because the oncogene (cfDNA) in the blood is very rare. However, if the cancer is diagnosed at the considerably advanced stage 3 or 4, the diagnosis is irrelevant because treatment is difficult. High sensitivity is critical for PCR technology to be used in the diagnostic market.
Fortunately, PCR technology has also been rapidly advanced in terms of sensitivity over generations. With the first-generation PCR in the past, ‘qualitative analysis’ was possible, and ‘relatively quantitative’ analysis was possible with the second-generation real time PCR. On the other hand, the third-generation 'digital PCR' makes 'absolute quantification' possible without quantified standard materials used for the existing second-generation PCR, and secured sensitivity enough to detect '0.001%' of the gene in samples.
CEO Lee explained that the sensitivity of digital PCR came from the difference in the sample analysis method. While the existing second-generation PCR, currently used for diagnosing COVID-19, analyzes one sample solution, the digital PCR divides one sample solution into tens of thousands to hundreds of thousands of samples and proceeds to gene amplification and analysis. Though this process, it is possible to secure the sensitivity for the gene to be detected. Therefore, digital PCR technology is expected to be commercialized in various diagnostic fields. Accordingly, many global companies including RevoSketch jumped into the development of digital PCR devices.
CEO Lee mentioned Bio-Rad, a bio device manufacturer in the United States, as a competitor dealing with digital PCR devices and technology. For Bio-Rad’s digital PCR device, one set consists of two or three devices, and digital PCR is carried out in three stages: 'sample fractionation', 'sample amplification', and 'target fluorescence detection'. However, since an independent instrument is used for each PCR step, an additional process of transferring samples is required between steps. This increases the risk of sample contamination, and can lead to errors. He also pointed out that even with the high sensitivity, the penetration rate was quite low due to the high cost of each device and the additional system for the device.
Therefore, RevoSketch's goal was to develop digital PCR devices by prioritizing economic feasibility over competitor's products and by unifying the work procedure into a single device to reduce the possibility of contamination and errors. RevoSketch's digital PCR device 'digiQuark' is designed for conducting all digital PCR processes in one device. CEO Lee said, “With digiQuark, we have reduced the cost of purchasing the equipment and system for each stage. It prevents sample contamination and errors in PCR results because no additional step is needed to transfer samples.” He also explained, “Additionally, the PCR device itself is small in size, and can be moved easily.”
How does RevoSketch's digital PCR device, digiQuark, work? RevSketch implanted ‘sdPCR (spinning digital PCR)’ centrifugation technology to digiQuark. After injecting a sample into a sample dish, the dish spins at a high speed, and the sample moves to the wall due to the centrifugal force. At this time, the sample is physically dispensed into the micro reaction wells on the wall. The sample of a small volume in the micro reaction structure is subjected to PCR cycle through thermal cycling on the wall of the dish.
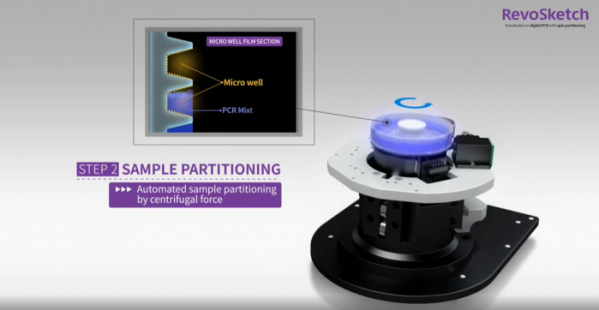
▲DigiQuark Sample Partitioning(RevoSketch)
DNA molecules in the micro reaction structure are scanned through a high-sensitivity optical module installed inside the device. The optical module inside the device can recognize up to 20 million pixels of fluorescence signals of genes in the micro reaction structure, and the scanned results are analyzed through RevoSketch's own software.
CEO Lee explained, “We analyze results by converting the fluorescence signals scanned through the high-resolution and high-sensitivity laser sensor in the device into digital information. Data obtained through up to five fluorescent channels can be handled by the conventional two-dimensional analysis. Based on six axes in the three-dimensional space, it is possible to analyze more complexed results.”
CEO Lee mentioned 'real time digital PCR' as another differentiating point of RevoSketch. Competitor Bio-Rad's digital PCR device, after completing all the 40 cycles, performs only one scan at the detection stage (endpoint scan) to obtain fluorescence information on the target DNA. RevoSketch’s device goes through the detection process by scanning every cycle. Using the fluorescence information on the target DNA obtained from the micro reaction structure through AI, it is determined whether or not the amplification process was normal. This means that it is possible to distinguish between 'true positive' that can be used as real data and 'false positive/negative' that cannot be used.
For an example of this difference, CEO Lee mentioned the ‘rain problem’. When analyzing the results of real-time PCR, it is not clearly distinguished whether the actual data are ‘positive’ or ‘negative’. Therefore, unseparated data fall down like rain. This is the rain problem. To solve this problem, a threshold is used. CEO Lee emphasized that results of PCR could be different by up to 17% depending on the range of the threshold.
CEO Lee presented that amplification curves of the micro-response structure were obtained by digiQuark at every cycle and checked through an artificial neural network to solve the Rain problem. Therefore, no separate judgment line is used for the data analysis process, and results of samples even at extremely low concentrations are reliable.
Now RevoSketch has completed global approval for its devices and preparations for full-fledged production. In March, it received the Ministry of Food and Drug Safety certification and the European CE-IVD certification. In December of last year, it received the KGMP certification related to the production of medical devices. digiQuark is scheduled to be released in 2022.
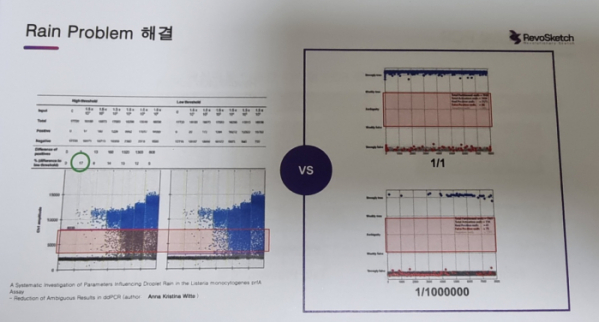
▲The rain problem of devices from Bio-Rad and RevoSketch







