기사본문
DCNbio, the “Three Approaches” to the Market
입력 2020-09-15 16:42 수정 2020-09-15 16:42
by Jongwon Jang

DCNbio is an exclusive company specializing in the marketing of three products including ▲Yeast-Drugs, ▲Microbiome Drugs, and ▲Functional Probiotics.
① Challenging Localization of the ‘Saccharomyces boulardii’
DCNbio targets the market of yeast-drug products. The company challenges the localization of ‘Saccharomyces boulardii’, currently produced and globally distributed by the French company, BIOCODEX, exclusively.
In cooperation with the “Korea Research Institute of Bioscience and Biotechnology”, DCNbio currently progresses towards the development of the production process wherein high-concentration culture of ‘Saccharomyces boulardii’ is enabled through the optimization of the fermentation and drying of ‘Saccharomyces boulardii’. President Lee stated, “… We have set-up the entire process for the mass production of yeast formulations which is under test with production of prototypes …”, “… the processes embracing culture of yeast and post-treatment of yeast formulations will also be developed for the higher content of viable microbes…”.
② Development of Yeast-Drugs and Microbiome New-Drugs
DCNbio intends for the development of the ‘pharmabiotics’ by grafting the technologies of either genetic recombination or killed organism etc., other than probiotics products of viable microbes, to the existing ‘Saccharomyces boulardii’, currently exploited as medicine for intestinal disorders. Through this approach, the company challenges the development of the microbiome therapeutic agents by which the medicinal efficacy of conventional product, limited to the extent of intestinal disorders, will be expanded to the amelioration of metabolic diseases or disorders in the central nervous system.
DCNbio probes for candidate strains capable of healing Parkinson’s disease based on the studies on ‘Gut-Brain Axis’, wherein the close connection between the gut and brain via signal transmission, mediated by intestinal microorganism, was proposed. In collaboration with the ‘Digestive Disease Research Institute (DDRI)’ in the ‘School of Medicine of Won Kwang University’, the studies on characteristics of intestinal microorganisms of Korean patients suffering Parkinson’s disease is in progress, from which the company expects to identify strains specific to Parkinson’s disease for the joint research with the ‘Korea Research Institute of Bioscience and Biotechnology’ intending for the development of the ‘pharmabiotics’ customized for ther characteristics of Korean patients.
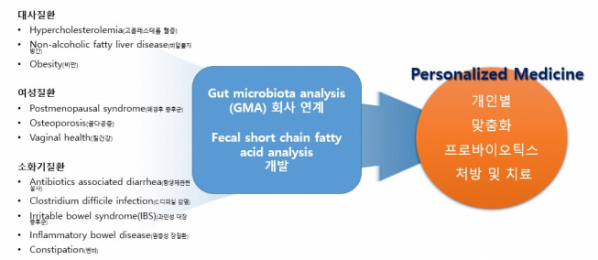
③ Development of Evidence-based ‘Probiotics’ Customized for Diseases-Individuals
DCNbio also promotes the development of the ‘probiotics’ fit for each kind of diseases. Among the diseases, the company targets the development of probiotics customized for patients suffering from metabolic diseases (hypercholesterolemaia, non-alcoholic fatty liver, obesity), females’ diseases (postmenopausal syndrome, osteoporosis, vaginal health), and digestive diseases (antibiotic-associated diarrhea, irritable bowel syndrome, inflammatory bowel disease, constipation) etc. Simultaneously, the company established the strategic relationship with the Irish company, ‘PrecisionBiotics’, to introduce excellent probiotics products of demonstrated efficacy to the domestic market. In particular, the ‘launching’ of the probiotics products of the family of 'Psychobiotics', improving mental symptoms such as depression, anxiety, and sleep disorder as well as stress by normalizing the balance of intestinal bacteria, is in progress.
DCNbio envisions the provision of the probiotics products customized for diseases and/or individuals through a platform associated with a company of GMA (Gut Microbiota Analysis), and has started the development of the diagnostic kit enabling the assessment of changes in the amount of short-chain fatty acid contained in the feces of individuals at home, clinics, drug stores, or specialty stores of probiotics etc.
President Lee of DCNbio stated, “… Currently, we are planning the invitation of external investors. Upon completion of the fund-raising, the laboratory of our company will be expanded to start the screening and pre-clinical trial experiment of functional strains, and then the clinical trials will follow.”







