기사본문
In the Grey Area of PD-1, Tecentriq Demonstrates Improved PFS in EGFR Mutation Lung Cancer
입력 2023-10-30 09:40 수정 2023-11-12 07:55
by Sungmin Kim

▲Sungmin Kim/Biospectator
Until now, EGFR or ALK mutations in lung cancer were considered an unknown area that immunotherapy drugs could not target. There have been many attempts, but have failed repeatedly.
However, the results of the ATTLAS phase 3 clinical trial, which may change this perspective, were announced as a late-breaking abstract (LBA) by the European Society of Medical Oncology (ESMO 2023) on the 21st, the first day of the conference. The presentation was conducted by a team led by Professor Ahn Myung-joo of Samsung Medical Center (SMC), and Lunit participated as a joint research institute to analyze biomarkers. At the same time, the results of phase 3 of ATTLAS were published in the Journal of Clinical Oncology (IF 50.739) as an international academic journal published by the American Society of Clinical Oncology (ASCO).
Previously, patients with EGFR mutated non-small cell lung cancer were first prescribed with EGFR TKI such as Tagrisso as the first-line treatment, and patients with ALK translocation will also be treated with ALK TKI. These targeted treatments improve the duration of progression-free survival (PFS) for up to several years in patients with the mutation, and show high drug response rates; the problem is refractory and almost all patients will relapse.
In other words, there is still an unmet need for post-TKI patients who have been treated with TKIs. US Merck (MSD) and BMS have also tried until recently to bring the remaining areas of PD-1 immune checkpoint inhibitors in the most important non-small cell lung cancer market, but failed.
First of all, last year, BMS administered PD-1 Opdivo and platinum-based chemical anticancer drugs to non-small cell lung cancer patients who had failed to treat from EGFR TKIs, but failed to improve the patient's PFS and overall survival period (OS). Merck also attempted to administer PD-1 Keytruda and platinum-based chemotherapy in phase 3 clinical trials of KEYNOTE-789 for non-small cell lung cancer patients who are refractory to EGFR TKIs. It failed because it only confirmed the trend of improving PFS and OS but did not reach statistical significance. As a result, platinum-based chemotherapy remains a standard treatment option for patients who are refractory to the first-line treatment EGFR TKI.
ChanYoung Ock , Chief Medical Officer (CMO) of Lunit, said, "In the current treatment of non-small cell lung cancer, if there is no EGFR or ALK mutation, immune checkpoint inhibitors are administered alone or with chemo depending on PD-L1 positivity. The administration of chemotherapy drugs alone is now being pushed out of treatment options.” "On the other hand, patients with EGFR or ALK mutations receive targeted therapy as a first-line treatment, then consider a platinum-based treatment as a second-line treatment, followed by a single immunotherapy agent”, he added.
However, he explained, "It is still in a gray area how to use immunotherapy." In the background, the leading runners, Keytruda and Opdivo, excluded non-small cell lung cancer patients with EGFR and ALK mutations from the approved clinical trial of the first-line treatment setting, and are also excluded from the approval label. Basically, there was also mechanistic evidence that if there is an oncogenic driver that strongly causes cancer, immune checkpoint inhibitors will not respond well due to low immunogenicity.
However, Roche's approach was different, conducting clinical trials to include a wider patient population. Roche designed the clinical trial so that patients with EGFR or ALK mutations can also be registered while conducting the IMpower150 Phase 3 clinical trial, which evaluates PD-L1 Tecentriq as a first-line treatment for non-small cell lung cancer that has not previously received chemotherapy. Non-small cell lung cancer patients with EGFR mutation were recruited if they relapsed or were refractory to previous EGFR TKI. However, the phase 3 primary endpoint analysis was limited to wild type patients without mutations, and patients with EGFR or ALK mutations were excluded.
Roche also attempted co-administration with the VEGF antibody ‘Avastin’. Avastin can increase the activity of chemotherapy and immunotherapy by increasing lymphocyte migration into the tumor microenvironment (TME) while inhibiting VEGF, which is involved in neovascularization, and is a mechanism to restore VEGF-mediated immunosuppression. In previous EGFR-mutant non-small cell lung cancer clinical trials, it was known that inhibiting VEGF in addition to EGFR TKI had the benefit of improving PFS.
As a result of key subgroup analysis, Roche announced in the Lancet in 2019 that positive results were confirmed when Tecentriq, Avastin, and chemotherapy were administered in combination to patients with non-small cell lung cancer with EGFR mutations (doi: 10.1016/S2213 -2600(19)30084-0). In patients who had previously received EGFR TKI, Tecentriq + Avastin + chemotherapy (carboplatin/paclitaxel) increased both PFS and OS compared to the control group Avastin + chemotherapy (HR = 0.42 and HR = 0.39, respectively). However, this is the result of a subgroup analysis, and only 10% of EGFR mutation patients participated in the clinical trial, so it did not lead to an approved label.
However, as Merck and BMS failed in the phase 3 clinical trial conducted with this strategy, the question of whether immunotherapy treatment is meaningful in patients who received EGFR TKI seemed to have fallen into limbo again and lost interest.
CMO Oak added, “The clinical results did not reach a consensus on whether immunotherapy would be helpful in these patients, when it should be administered, and which patients would respond.”
Finding clues from ATTLAS phase 3 clinical trial
With the results of the domestic ATTLAS phase 3 clinical trial led by Professor Myung-joo Ahn as clinical director, the embers that had been extinguished are being revived again.
This clinical trial is the first randomized phase 3 clinical trial result to see the benefits of immune checkpoint inhibitors in lung cancer patients who received EGFR TKI. The clinical trial was conducted under the leadership of Samsung Medical Center in Korea after receiving Tecentriq from Roche. Based on regulatory agency standards, in Korea, as of 2019, the combination of Tecentriq, Avastin, and chemotherapy drugs could be prescribed for patients with previous EGFR or ALK mutations with marketing approval.
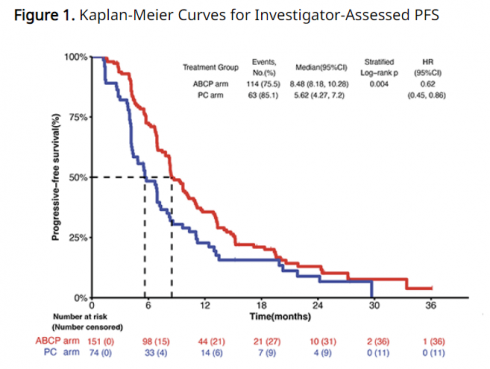
This ATTLAS phase 3 clinical trial was conducted on 228 patients with non-small cell lung cancer with EGFR or ALK mutations who had previous experience receiving targeted therapy, and the combination of Tecentriq + Avastin + chemotherapy (carboplatin/paclitaxel) was compared with Avastin + chemotherapy (pemetrexed + carboplatin or cisplatin) (NCT03991403). The primary endpoint was set as PFS, and the secondary endpoints were OS, ORR, and duration of drug response (DoR).
More than half of the patients who participated in the clinical trial had previously received 1st and 2nd generation EGFR TKIs, and the remaining patients had experience with 3rd generation EGFR TKIs. Among these, the number of patients who received third-generation EGFR TKI as first-line treatment was 8% in the Tecentriq group and 13% in the control group.
Clinical results confirmed that the PFS of Tecentriq co-administration was 8.48 months, a significant improvement compared to the control group of 5.62 months. The result was that the patient's risk of disease stage progression or death was reduced by 38% when administered in combination with Tecentriq (HR 0.62, p=0.004). Additionally, the higher the PD-L1 expression, the greater the PFS benefit. The HR values were 0.47, 0.41, and 0.24 for PD-L1 expression criteria of 1% or more (≥1%), 10% or more (≥10%), and 50% or more (≥50%), respectively.
▲ESMO
This difference was also observed when applying Lunit's artificial intelligence (AI)-based biomarker. The research team applied Lunit SCOPE IO, which calculates the inflammation score by analyzing the spatial distribution of tumor-infiltrating lymphocytes (TIL) in cancer tissue, and set the cut-off at 20%. As a result, when the inflammation score was 20% or more, the Tecentriq combination treatment group showed a 79% improvement in PFS compared to the control group (12.91 months vs. 4.86 months, HR 0.21, p=0.002). For the score lower than 20%, the difference in PFS between the two groups was not observed. (HR 0.82).
CMO Oak said, “When applying the inflammation score of Lunit Scope IO, we were able to confirm that the difference in PFS between the two groups was clearly greater depending on higher or equivalent PD-L1 expression,” adding, “Also, in the case of PD-L1 expression, immunohistochemistry (IHC) analysis should be conducted by obtaining additional tissue samples, while AI-based TIL analysis has the advantage of securing a larger number of samples because of its mechanism that it can be analyzed using existing cancer tissues (H&E).".
Regarding the significance of these results, CMO Oak said, “When non-small cell lung cancer patients have EGFR or ALK mutations, their immunogenicity is low, which makes it difficult to see an advantage of administering IO(immuno-oncology) drugs alone compared to chemotherapy.” He added, “In that sense, we need a biomarker to select which patients to display such advantages. In the phase 3 clinical trial of Keytruda conducted on patients who previously received EGFR TKI, a similar survival benefit was observed regardless of the level of PD-L1 expression,” he explained. In the KEYNOTE-789 phase 3 clinical trial, there was no difference in OS according to PD-L1 expression (PD-L1 TPS≥50% HR 0.84, TPS<50% HR 0.85).
In that respect, you can apply Lunit Scope IO. He added, “We are currently discussing applying Lunit Scope IO to previous clinical failure cases.”
In the subgroup analysis, which will be noted in this phase 3 clinical trial, differences depending on EGFR mutation type were also observed. In patients with the EGFR L858R substitution mutation, which is known to have a poor prognosis, the PFS HR of 0.52 when administered with Tecentriq was more evident than the PFS of 0.69 observed with the exon 19 deletion mutation. In addition, Tecentriq also showed improvement in the ORR efficacy index (ORR 69.5% vs 41.9%, 0<0.001).
However, there was no difference in OS between Tecentriq co-administration and the control group (20.63 months vs 20.27 months, HR 1.01, p=0.975). The safety profile showed a higher incidence of grade 3 or higher side effects in the Tecentriq combination treatment group compared to the control group (35.1% vs 14.9%). The most common side effects from the Tecentriq treatment group were peripheral neuropathy, hair loss, and muscle pain.
Professor Ahn, who is in charge of this clinical trial, explained, “The combination of Tecentriq, Avastin, and chemotherapy significantly improved PFS statistically and clinically, and ORR also increased compared to the control group,” adding, “It should be considered a reasonable treatment option.”
Future competition will also be taken into consideration. Antibody-drug conjugate (ADC) and Tagrisso combination administration are being attempted for patients with non-small cell lung cancer who have relapsed or refracted after receiving previous EGFR TKIs. In this ESMO, the results of Phase 3 clinical trials will be announced, which showed the combination of J&J's EGFRxMET double antibodies "amivantamab" and "laseratinib" has an advantage over chemotherapy.
관련기사
- Lunit’s ‘expansion’, ‘TIL biomarker’ and its strategy for PD-1 market
- Lunit's Efforts to Transform AI Breast Cancer Screening Featured in The Lance...
- Lunit participates in the ‘Vision 2030 SEHA Virtual Hospital’ project in Saud...
- CXR-AID, Powered by Lunit, Nets Japan Nod for Reimbursement, Accelerating Mar...
- Guardant Health bets $26M on AI diagnostics-focused Lunit







