기사본문
IMBDx challanges Liquid Biopsy with NGS-Based Multi-Markers
입력 2020-01-22 11:14 수정 2020-01-22 11:14
by Jongwon Jang
IMBDx, a domestic bio start-up company, has undertaken the challenge to enter the global liquid biopsy market in 2020. The domestic bio start-up company is headed by Prof. Kim Tae-You of Seoul National University, Director Kim Hwang-Phill of the research center, and CEO Peter Moon, management specialist.
IMBDx plans to join the liquid biopsy market based on the NGS and multi-markers. The IMBDx applies the strategy of differentiating itself from competitors through bioinformatics engine technology including highly efficient biochemistry pretreatment technology applying self-developed reagents, sequencing technology, and deep learning.
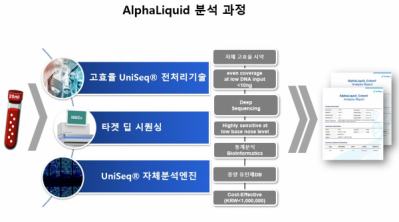
As of now, the company has secured the analysis capacity with 95% sensitivity and 0.5% specificity in a small amount of more than 10ng of cfDNA and has initiated R&D efforts to improve the LOD to 0.1%. The bio-company has secured patents for noise removal technology in the analysis of the cfDNA gene analysis and for self-developed protocols.
CEO Moon said, “The company has secured and is now confirming technologies for simultaneous analysis of SNV, Indel, CNV, Fusion, etc. and also methylation technology for adding epigenetic markers. It is currently developing neoantigen mapping technology and verifying theses technologies at home and abroad to accelerate their commercialization.”
IMBDx has completed the development of AlphaLiquid-Colon products used for monitoring the metastasis and recurrence of cancers by analyzing 10 genes and plans to apply for domestic approval this year. Additionally, the company plans to develop and verify next-generation products such as AlphaLiquid 100, AlphaLiquid 1000 by increasing the number of cancer-related genes to be analyzed to 106 and 1,084 types.
AlphaLiquid-100 is expected to compete with 'Guardant360' developed by Guardant Health which identifies mutations in 73 genes. AlphaLiquid-1000 identifies markers available for comorbid diagnosis and can perform cohort configuration and analysis to improve anticancer response rates. The company plans to develop its liquid biopsy platform into a service applicable to clinical trials in reflection of the needs of new drug developing companies.
IMBDx will start in full swing the "10K Alpha Project" to demonstrate the competitive edge of the AlphaLiquid series by targeting 10,000 cancer patients. "We aim to establish ourselves as a leading group in the domestic cancer diagnosis by securing products and data which can be actually used by medical staffs."







