기사본문
TiCARos Therapeutics, "Clinical study next year" of 'CLIP CAR-T' to strengthen immune synapses
입력 2021-11-25 09:49 수정 2021-11-25 09:49
by Yoonseok Suh

Jae Won Lee, CEO of TiCARos, said, “We are developing a CAR-T that has improved anticancer effects and reduced side effects by engineering T cells” and “We plan to enter a phase 1 clinical study for blood cancer by next year, and our ultimate goal is to develop a next-generation CAR-T that can be applied to solid cancers in the future”.
TiCARos Therapeutics is currently developing three CAR-T development platforms: CLIP CAR, Converter CAR, and Switchable CAR. In addition, TiCARos has established an antibody research team and is discovering new antibodies on its own.
TiCARos' lead pipeline is CD19 CLIP CAR-T 'TC011' with CLIP CAR(Clamping-based Immunological Synapse Potentiating CAR) technology that enhances the immune synapse of T cells and cancer cells. At the end of this year, TiCARos plans to submit a phase 1 clinical study plan(IND) of TC011 to the Ministry of Food and Drug Safety in Korea for diffuse large B cell lymphoma(DLBCL), a blood cancer, and initiate the clinical study next year.
TiCARos was founded in 2018 by Jae Won Lee, former CFO of Dinona, Kyungho Choi, Professor at Seoul National University College of Medicine, and Eun Young Choi, Professor at Seoul National University College of Medicine, and is developing CAR-T by transferring the exclusive license for the T cell enhancement technology developed by Professor Kyungho Choi while working at the National Cancer Center. TiCARos is currently operated under the sole CEO system, but will soon switch to a separate CEO system together with Professor Kyungho Choi. TiCARos has 20 researchers, including 15 with master's and doctoral degrees, and is continuously recruiting researchers.
TiCARos' short-term goal is to enter a phase 1 clinical study for lead pipeline TC011 and attract Series C investments by next year. In the mid- to long-term, TiCARos plans to expand indications to solid cancers, advance platform technologies such as Switchable CAR-T, and promote an initial public offering(IPO). Biospectator listened to TiCARos' platform technologies and pipelines.
◆ Differentiation from existing CAR-T.."Increased immune synapses, increased T cell activity, increased anticancer effect"
First, let's look at the structure of CAR. The CAR structure consists of an scFv domain that recognizes a tumor antigen outside the cell, a hinge, and a T cell activation signal transduction site through a transmembrane domain(TM). In particular, in the transmembrane domain, CD28, 4-1BB, OX40, etc. are used as co-stimulatory molecules for T cell activation, and CD3ζ is used as a stimulatory molecule.
Looking at the hinge/co-stimulatory molecule/stimulatory molecule structure of the currently approved CAR-T treatments, 'Kymriah' has the structure of CD8a/4-1BB/CD3ζ, 'Yescarta' has the structure of CD28/CD28/CD3ζ, and 'Breyanzi' has the structure of CD28/4-1BB/CD3ζ(doi: 10.1158/2159-8290.CD-15-0583). CEO Lee said, “Most of the current CAR structure improvement to enhance the anticancer effect has been to add or replace signal transduction sites such as CD28 and 4-1BB” and “We have differentiated ourselves from the existing method by focusing on the immune synapse, which is the contact surface between T cells and tumor cells, to enhance the anticancer effect”.
In particular, the existing CAR-T treatment is considered a game changer as it has a high ORR of about 34-66% for blood cancers such as malignant B-cell lymphoma, but it has a limitation in showing a low response rate in solid cancers. The reasons for the low efficacy of CAR-T in solid cancers include △Difficulty in selecting tumor-specific antigens with high efficacy and safety due to heterogeneity of solid cancers △Insufficient penetration of CAR-T into solid cancers △Tumor Micro Environment(TME) creates an immunosuppressive environment.
Recently, to overcome this, the development of CAR-T using various antigens such as EGFR and mesothelin, and combination therapy to increase the immune response by modulating immuno-oncology and TME are being studied.
◆ ‘CD19 CLIP CAR-T TC011’ with enhanced affinity between CAR-T cells and tumor cells.. ”Entering a clinical study next year”
As a platform technology that strengthens these immune synapses, TiCARos has three platform technologies, such as CLIP CAR, which enhances the affinity between CAR-T and tumor, Converter CAR, which enhances T cell activation, and Switchable CAR, which controls drug toxicity using adapters, and its own antibody engineering technology that can develop new targets.
First, CLIP CAR is a technology that increases the tumor removal effect by increasing the immune synapse, which means the adhesion of CAR-T cells to tumor cells, and TiCARos is developing the lead pipeline CD19 CLIP CAR-T ‘TC011’ by applying this. CLIP CAR is a technology that increases the anticancer effect by modifying the hinge region of the CAR structure to increase the adhesion of CAR-T cells to tumor cells.
Using a model in which lymphoma cells were injected into immune-deficient NSG mice, TiCARos compared and evaluated the anticancer effect by administering m19BBz and TC011, which are CAR-Ts of the same type as that of Kymriah. As a result, in the control group, the cancer spread throughout the body from the 14th day, and all died on the 21st day. On the other hand, the mice administered with m19BBz showed a tendency to decrease tumor cells on the 14th day compared to the control group and increase the tumor cells on the 21st day. On the other hand, in mice administered with TiCARos’ TC011, the cancer began to disappear from the 14th day of administration of tumor cells, and was hardly observed on the 21st day.
CEO Lee said, “Currently, toxicity studies are in progress at the GMP level, and the lab study confirmed that there was no effect on the toxicity and weight change of the mouse model” and “We expect that there will be no toxicity problems as it survives for 150 days after TC011 administration”.
Based on these results, TiCARos is conducting a study with the goal of entering a phase 1 clinical study for TC011 next year, and plans to submit a phase 1 clinical study plan(IND) to the Ministry of Food and Drug Safety in Korea at the end of this year. CEO Lee said, “We have a plan to conduct a phase 1 clinical study at Seoul National University Hospital with a clinical study design similar to that of a leading group in Korea” and “We want to give hope to patients suffering from cancer”.

◆ Immune tolerance overcoming CTLA4-CD28 CAR-T.. "Anticancer effect even at a low dose in a blood cancer model"
In addition to CLIP CAR technology, TiCARos is also studying CAR-T with Converter CAR technology that increases T cell immune function by converting T cell immune checkpoint inhibitory signal into activation signal.
For normal T cell activation, in addition to recognizing antigens by binding of T cell surface receptor(TCR) and MHC of APC, activation of lower signal through CD28, a T cell stimulator, is required. CD28 binds to B7, a ligand on the surface of APC, to activate T cells, but when over-activated, CTLA4 expression, which has 20 to 100 times higher B7 affinity than CD28, increases on the T cell surface and inactivates T cells. CEO Lee said, “Co-founder, Professor Choi, has developed a chimera in which the signal transduction site of CTLA4 in T cells is replaced with CD28 based on this”.
To prevent immune tolerance, in which the anticancer effect decreases as T cells are inactivated, TiCARos replaced the lower signal transduction site with CD28 to activate the immune response while maintaining the B7 receptor binding of CTLA4 and APC(CTLA4-CD28, CTC28). This is a concept that increases the activity of T cells and increases the anticancer effect.
Common CTLA-4 antibody suppresses the inactivation of T cells and strengthens the immune response against cancer cells to obtain anticancer effects, causing side effects due to excessive immune responses. CEO Lee said, “We are developing a CAR-T cell treatment that enhances anticancer effects and reduces side effects with CTC28 CAR-T cells introduced with CAR that targets cancer antigens and CTC28 that activates T cells”.
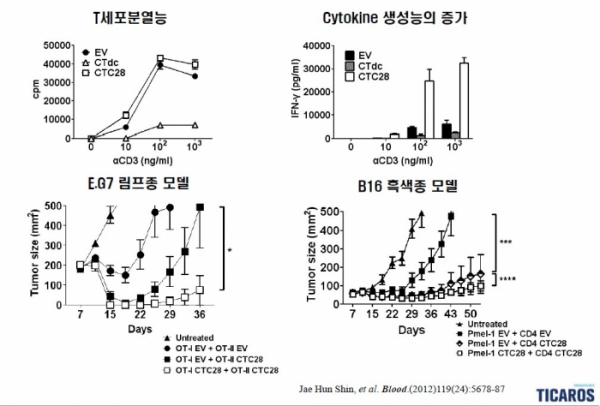
Could this approach enhance the anticancer effect? To verify this, TiCARos verified the anticancer effect using a lymphoma mouse model and a melanoma mouse model, and as a result, it was confirmed that the tumor size was effectively decreased, and the T cell differentiation and immune cytokine secretion were increased(doi:10.1182/blood-2011-09-380519).
Specifically, TiCARos administered CTC28-introduced T cells into a mouse model and analyzed the tumor size every 7 days. As a result, the mice administered with CTC28-introduced CD4 T cells showed a tendency for tumors to almost disappear and then recur within 7 to 15 days after administration, and in mice administered with cells in which CTC28 was introduced into both CD4 and CD8 T cells, tumor cells almost disappeared for about 21 days.
Similarly, the melanoma model showed a reduction in tumor size. When CTC28-introduced T cells were administered, the tumor size decreased from 100 ㎟ while the tumor size increased from 100 ㎟ to 500 ㎟ in the control group over about 21 days, showing a therapeutic effect, and then gradually recurred and slowly increased to about 100 ㎟ at the time of 50 days.
In addition, the anticancer effect was confirmed in a mouse model administered with blood cancer cells using CTC28 CAR-T cells of this structure. As a control group, m19BBz, a CAR-T of the same type as that of Kymriah, was used, and as an experimental group, ‘CTC28 CAR-T’ with converter technology was used.
First, when m19BBz was administered at a dose of 5x10^6, the tumor size was effectively decreased, confirming the anticancer effect. Next, TiCARos administered m19BBz, which was reduced to 1/10 of the dose, and CTC28 CAR-T at a dose of 5x10^5. As a result, tumors recurred from about 20 days after administration with m19BBz, whereas tumors did not recur even after about 40 days after administration with TiCARos’ CTC28 CAR-T.
In addition, lymphoma cells were administered into the immundeficient NSG mouse model and then m19BBz and CTC28 CAR-T were administered. The control group died 2 weeks after administration, and the mice administered with m19BBz showed a decrease in tumor size 1 week after administration, and then the tumor spreads from 2 weeks after administration. On the other hand, CTC28 CAR-T had a reduced tumor than m19BBz from 1 week after administration, and almost no tumor was observed after 2 weeks.
CEO Lee said, “Actually, the Converter CAR-T showed excellent anticancer effect in the mouse model” and “Currently, we are developing CLIP&Converter CAR-T 'TC012' in the preclinical stage, which has improved anticancer effect by combining it with CLIP CAR technology”.
In addition, TiCARos is developing a Switchable CAR-T to overcome the toxicity issue that appears in the existing CAR-T cell treatment. Most tumor surface antigens are not specifically expressed in tumors but are also expressed at low levels in normal cells, resulting in toxicity.
TiCARos presented an on/off target toxicity avoidance approach that controls toxicity to normal cells by controlling the amount of adapter antibody by making an antibody as an adapter instead of direct binding of CAR-T cells to tumor antigen. TiCARos engineered antigens such as CD19 and CD40 that target cancer cells to express specific epitopes, and uses CAR-T cells that bind to these epitopes.
As a result, they bind in the order of cancer cell-adapter antibody-CAR-T cell treatment, and as the adapter antibody binds more in tumor cells than in normal cells, it is a concept to control toxicity by adjusting the dose. TiCARos has discovered two new antibodies for adapters and aims to enter preclinical studies next year.
In addition, as a strategy to overcome the heterogeneity of tumor antigens expressed by cancer cells, TiCARos is developing CAR-T applicable to solid cancers by discovering new targets expressed in tumor cells and vascular endothelial cells in tumors.
CEO Lee said, “Although optimization is still needed, we confirmed excellent anticancer effects as a result of early studies in mouse models” and “We expect that it can be applied to lung cancer, ovarian cancer, breast cancer, and liver cancer, and we plan to proceed with lung cancer and liver cancer as the first target”.
관련기사
- OliX Pharmaceuticals, 'GalNAc-siRNA platform' competitiveness against HBV∙NASH?
- PeLeMed Develops the Low-Molecular Compound, What Are the Advantages of 'PeLe...
- JW Bioscience signs with Immunovia for the patent ‘biomarker of pancreatic ca...
- AItheNutrigene, ‘Paper Biochip’ New Approach of Self-Rapid Diagnosis
- Geninus, Single Cell Technology for precision medicine-from dignosis to drug ...







