기사본문
Abclon, at a turning point, three scalability of ‘antibody technology’
입력 2023-04-27 09:19 수정 2023-04-27 09:19
by Sungmin Kim

This year marks a turning point for Abclon as it transforms into a completely different company. The first interim results of phase 1 clinical trials of CAR-T candidates based on Abclon's antibody technology released at the American Association for Cancer Research (AACR 2023) next month, and the results of phase 2 clinical trials of anticancer drug candidates for gastric cancer are expected to be derived in the first half of this year. Another step forward is expected 14 years after Abclon was established with confidence in antibody technology.
If you pick a word that can represent the Abclon so far, it is their 'standard tactics'. The basis for Abclon’s antibody technology is CEO Lee Jong-seo's know-how in producing 80,000 antibodies for 20,000 human proteins during the Human Protein Atlas project with researchers from the Royal Swedish Academy of Sciences for the past 20 years since 2003. CEO Lee co-founded Abclon in 2010 to develop such antibody technology as a treatment with Professor Carl Erik Mathias Uhlén, the project's general manager.
As the recognition that ‘Abclon makes the best antibody’ has accumulated, it has been transformed into what it is today. Since 2016, Abclon has signed an antibody discovery and bispecific antibody joint research with Yuhan Corporation four times, and licensed out(L/O) HER2 antibody for CAR-NK production in 2019 to GC Cell, the original developer of Artiva therapeutics technology, which signed a CAR-NK discovery deal with Merck (MSD) of the United States. Along with this, in the mid-2010s, when the clinical results of CAR-T first received attention from the global industry, Abclon received a joint research proposal and expanded the scope of research and development into CAR-T treatments.
Lee Jong-seo, CEO of Abclon, said, “Depending on which epitope is used to produce an antibody treatment for a specific disease, it can have a completely different mechanism of action. As a result, the company started with the idea that it could have a differentiated therapeutic effect from existing treatments. This year, a global pivotal study of the HER2 antibody 'AC101 (HLX22)', which technology was transferred to Henlius in China, is planned to be conducted, and the antibody treatment developed in Korea is approaching the commercialization stage. And it is very overwhelming with joy that the commercialization is getting closer.”
Since last year, data showing the potential of Abclon's strategy have begun to emerge as clinical results. Henlius announced in its earnings report in the first half of last year that it estimated that the HLX22 combination therapy would produce an overall response rate (ORR) of 85% or higher in the phase 2 clinical trial for the first-line treatment for HER2-positive gastric cancer. The company announced plans to pursue a global pivotal study for the first-line treatment for gastric cancer with HLX22 combination treatment.
Here, as the first announcement of the phase 1 clinical interim results of CD19 CAR-T 'AT101' at AACR 2023 to which Abclon applied a new epitope discovered by itself, the industry's attention is focused on whether it will produce clinical results differentiated from the existing CD19 CAR-T.
This is the reason why Abclon's scalability is expected in the future. Abclon is based on 'NEST (Novel epitope screening technology)', an antibody discovery platform that searches for new epitopes, and 'Affibody' technology, which is 1/25 the size of antibodies, which is expanding to ▲new epitope antibodies and bispecific antibodies ‘AffiMab' and other antibody drugs ▲CAR-T ▲Switchable CAR-T
At the same time, Abclon continues to provide antibody service to partners such as antibody-drug conjugate (ADC) and allogeneic CAR-T. Abclon posted 2.9 billion won in sales last year as an antibody service. In Korea, it has signed MOUs with ADC specialist Biotech In2cell and Aptis, and CRISPR companies, Toolgen and Gplus.
Scalability① - New epitope differentiation 'clinical proof of its concept'
Abclon's lead project is 'AC101', a new epitope HER2 antibody derived from the NEST platform. CEO Lee said, “We started by establishing a company and making HER2 antibody treatments. At the time of development, there was already a blockbuster drug called 'Herceptin (trastuzumab)', so I was asked why I was developing it." "Abclon discovered HER2 antibodies by focusing on gastric cancer, which is more common in Asia than in the West, from the beginning. Breast cancer was considered as the next priority,” he explained.
Looking at the background of Abclon's strategic decision at the time, Herceptin received marketing approval in the United States in 1998 as a treatment for HER2-positive breast cancer, and then expanded its indications to postoperative (adjuvant) therapy. However, it was only in 2010 that it was marketed as a gastric cancer treatment. According to the updated overall survival (OS) data of the ToGA, phase 3 clinical trial, which supported an approval from the US Food and Drug Administration (FDA) for Herceptin and chemotherapy combination as a first-line treatment for HER2-positive gastric cancer, the combination therapy showed a longer patient survival than conventional chemotherapy. It increased OS by about a month and a half (13.1 vs 11.7 months), which was seen as a limited result that did not meet the encouraging benefits seen with Herceptin in breast cancer.
Meanwhile Roche is starting to develop 'Perjeta (pertuzumab)' to respond to Herceptin's patent expiry. Perjeta is an antibody that targets HER2 like Herceptin, but has a different epitope with different mechanism of action from Herceptin (domain IV). Perjeta works as a new mechanism to inhibit HER2/HER3 dimer formation by targeting domain I.
CEO Lee said, “Even though Herceptin and Perjeta were discovered in the same year, they were released later to respond to the release of a Herceptin biosimilar. Perjeta is developed to use as a combination treatment along with Herceptin. When the two drugs are administered together, the response rate is higher than that of Herceptin alone. Perjeta is priced higher than Herceptin, which has already been released.”
Perjeta is also a drug that has gone through twists and turns in the development process. Genentech, the original developer, conducted phase 2 clinical trials with Perjeta alone in the mid-2000s, but failed one after another in solid cancers such as breast, prostate and ovarian cancer and eventually stopped development. Then, when Roche acquired Genentech in 2009, it promoted clinical development of combination administration, and in 2012, it was launched as a treatment for HER2 breast cancer, steadily expanding the market as an initial treatment.
However, the Herceptin and Perjeta combination administration strategy could not overcome the barrier of gastric cancer. In 2017, Roche announced the failure of JACOB phase 3 clinical trial for the Perjeta combination therapy for gastric cancer, and eventually failed to be marketed as a gastric cancer treatment, and the HER2 ADC 'Kadcyla', which combines Herceptin with anticancer drug, also failed in gastric cancer.
Abclon started with a similar idea, but from the beginning of development, it had a different goal of treating gastric cancer, which is a highly common disease in Asian patients. AC101 found by Abclon binds to the HER2 extracellular domain I like Herceptin, and according to CEO Lee, its mechanism is to completely block HER2 signal transduction by clamping the opposite side of the site where Herceptin binds.
Abclon confirmed that the combined administration of AC101 and Herceptin in a preclinical gastric cancer model had a higher inhibitory efficacy in tumor growth than the single administration of Herceptin or the combined administration of Perjeta. CEO Lee said, "Based on this data, I visited leading biosimilar companies at home and abroad to propose commercialization. And the strategy worked for Henlius, the largest biosimilar company in China at the time.”
Finally, in 2016, Abclon licensed out the rights to AC101 in China to Henlius for $16.5 million, including a $1.5 million down payment. Henlius is a company that launched rituximab biosimilar (brand name: Hanlikang) as the first biosimilar in China in 2019. Henlius released a total of four biosimilars the following year, including a Herceptin biosimilar in China, and while expanding its portfolio with new drugs. It also launched its own PD-1 antibody last year (as of March 2023).
Since then, Henlius has secured data that Herceptin biosimilar 'HLX02', which is being developed by AC101 (HLX22), produces synergistic effects in gastric and breast cancer models through preclinical trials, and expands the deal to secure worldwide rights for AC101 in 2018. It was supported by the results reproduced from the preclinical data previously confirmed by Abclon. According to the additional contract, Abclon signed a deal worth a total of $40 million, including a $10 million down payment from Henlius, and sales royalties are separate.
Henlius accelerates clinical development as it secures rights worldwide. In 2019, Henlius conducted phase 1 clinical trials of AC101 as a monotherapy for patients with HER2-positive advanced solid cancer and confirmed its tolerability and safety. Then, in September 2021, it conducted a phase 2 clinical trial comparing AC101 + Herceptin + chemotherapy (XELOX) combination therapy with Herceptin + chemotherapy (XELOX) in the first-line treatment setting for 150 patients with HER2-positive advanced or metastatic gastric cancer. In addition, in October of last year, Henlius received approval for a Chinese phase 2 clinical trial protocol (IND) for the combination administration of AC101, its own PD-1, and Herceptin biosimilar in HER2 gastric cancer, and plans to expand clinical development.
Henlius is conducting the trial to evaluate doses of AC101 15 mg/kg or 25 mg/kg as a phase 2 clinical trial for gastric cancer, and proceeds in three groups including the control group (NCT04908813). The primary clinical endpoints were progression-free survival (PFS) and overall response rate (ORR). The clinical trial is expected to end next month, and accordingly, top-line results are anticipated to be announced within the first half of the year.
In a semi-annual report in August last year, Henlius introduced that AC101 showed "great efficacy" in the interim results of AC101’s phase 2 clinical trials for gastric cancer, and expressed confidence in its potential as an initial treatment for HER2 gastric cancer.
Henlius predicts an ORR of 85% or higher in the AC101-administered group in the phase 2 clinical trial setting for the first-line treatment of HER2-positive locally advanced or metastatic gastric cancer. The basis for this estimation is that a drug response (ORR 100%) was confirmed in both patients who had previously administered 15 mg of AC101, and an ORR of 77.3% was estimated from blind data. In other words, the epitope differentiation strategy of AC101 observed by Abclon in preclinical studies is presented with actual clinical results. Based on these data, Henlius plans to evaluate the combination of AC101 and its own PD-1 (serplulimab).
CEO Lee said, “Based on the positive clinical phase 1 and 2 results, Henlius plans to pursue a global pivotal study of AC101 this year. We expect that this will be the first HER2 antibody combined treatment in gastric cancer, where the previous combination treatment of Herceptin and Perjeta did not show any efficacy.”
Henlius believes that there is a sufficient possibility even considering the current state of competition. AstraZeneca-Daiichi Sankyo's 'Enhertu', which is rapidly changing the field of HER2 targeting anti-cancer drugs with high efficacy, has not yet started phase 2/3 clinical trials in the first-line gastric cancer treatment setting. It received marketing approval as a third-line treatment for gastric cancer patients with prior experience with HER2 drugs.

The follow-up program focuses on the technology of ‘AffiMab (Affibody-fused bispecific antibody)’, a bispecific antibody in which an affibody is applied to an antibody. Abclon previously signed a license deal with Affibody, a Swedish biotech company that owns the Affibody platform, in 2013 to apply the company's Affibody technology to Abclon's antibody platform.
Affibody is an antibody mimetic without Fc, and has a size of 6kD, which is 1/25 of that of a monoclonal antibody. Swedish biotech Affibody is expanding its bispecific antibody and multi-antibody platforms, and taking advantage of the strengths of Affibody, this month signed a deal with Italy's Chiesi Farmaceutici to discover a new Affibody with a type of inhaler to develop a treatment for respiratory diseases.
Abclon discovered the TNFαxIL-6 bispecific antibody ‘AM201’, a candidate for rheumatoid arthritis treatment, as the first bispecific antibody program through joint research with Affibody since 2014. AM201 is in the preclinical stage, and in a rheumatoid arthritis animal model, AM201 showed higher efficacy (in inhibiting biomarker SAA) than the existing blockbuster TNFα drug.
Abclon is prioritizing the 4-1BB (CD137) bispecific antibody, which is a candidate for immunotherapy. Abclon discovered the EGFRx4-1BB bispecific antibody 'AM105' by applying the AffiMab platform, and AM105 is a drug with a mechanism that shows immunotherapy efficacy by specifically activating T cells mediated by 4-1BB in EGFR-overexpressing cancer cells. Abclon prioritizes colorectal cancer and evaluates its efficacy, and is securing differentiated data compared to competing drugs.
Scalability② – Extending the epitope strategy to CAR-T.. first phase 1 interim data at AACR
CAR-T is a new keyword that will represent Abclon in the future. The company's business expansion strategy may be questionable in that CAR-T is basically a gene/cell therapy product that manipulates immune cells, but the keywords are epitope differentiation along with the fact that antibody technology is used to produce CAR that T cells recognize cancer
CEO Lee said, “The year 2015 was a time when big events occurred one after another in the CAR-T field. At that time, the University of Pennsylvania’s medicine school, a leading CAR-T group, transferred CAR-T technology to Novartis and developed clinical results of the current 'Kymriah (tisagenlecleucel)'. Since then, Gilead Science has acquired Kite Pharma, and Juno Therapeutics has carried out Seattle Children's Hospital on its back, leading to a fierce patent dispute between them”, he explained.
It was the time when Abclon decided to expand CAR-T, and the trigger was a 'fateful meeting'. CEO Lee said, “In the mid-2010s, inquiries related to cell therapy continued to come in, and then in 2015, Seoul National University Professor Joon-Ho Jeong’s team found Abclon’s antibody technology and proposed a switchable CAR-T joint research.” “After much consideration, we decided that there were areas to be improved in the development of CD19 CAR-T, and we started full-scale CAR-T research.”
Abclon decided to approach the CAR-T development with a standard tactics. It was judged that it was important to find an epitope differentiated from the previously marketed CD19 CAR-T, and to secure an independent patent for the CAR structure, especially for commercialization while watching the CAR-T dispute over the past. However, it took more time than expected to discover a new CD19 CAR epitope that satisfies this requirement.
CEO Lee said, “All four CAR-Ts that have been marketed so far, including Kymriah, are applied with the FMC63 (CD19 scFv) CAR found in mice, so they have a complicated relationship in which they exchange royalties after a dispute over business rights.” “AbClon is a company with strengths in antibody technology, and has focused on analyzing patents and patent infringement (FTO) to secure its own CD19 CAR epitope and overseas business rights,” he said. AbClon registered a US patent for its self-discovered CD19 CAR ‘h1218 antibody’ in November of last year, completing its fourth patent registration following in Korea, Japan and Canada.
In this process, it unexpectedly ran into difficulties in the process of discovering new epitopes. According to CEO Lee, the discovery of the CD19 antibody was a “very unusual case.” Although Abclon has already had experience in making antibodies against 20,000 human proteins, the antibodies found by injecting antigens into existing animal models or phage display methods did not bind to human CD19 protein. This is the reason why it is interpreted that there may be still unknown parts of the structure/biology of CD19 scientifically.
At that point, Abclon changed its thinking. Rather, when antibody screening was attempted in chickens, which have the most heterogeneous immune system from humans, an antibody that binds to human CD19 was finally found, and the humanized antibody is now the h1218 antibody. Binding to a different epitope compared to existing FMC63 which targets exon 3-4 of CD19, h1218 has a similar affinity to CD19. h1218 clone binds to a membrane-proximal epitope of CD19 (exon 2 region K59-K63) thereby not competing with FMC63.
This different approach led to a collaboration with the University of Pennsylvania medicine school. CEO Lee explained, "While conducting a comparative study on how h1218 differs from FMC63 as it has a new epitope, a joint research proposal came from UPenn." Abclon has started joint research with Professor Marco Ruella of Cell Immunotherapy Division at UPenn since 2020, expanded the joint research last year, and is preparing to publish a paper on the research results.
Abclon developed CD19 CAR-T, ‘AT101’ by applying a 4-1BB-based second-generation CAR structure to a new epitope, and manipulated CAR-T using a lentiviral vector. CEO Lee said, “There are a total of four CD19 CAR-Ts that have been released to the US Food and Drug Administration (FDA) so far, and there is a reason why upgraded versions of drugs are being developed to continuously improve efficacy. However, there was no significant improvement in the antibody (FMC63), only signaling domain changes or manufacturing improvements.”

In in vitro and in vivo tests, Abclon confirmed that AT101 has at least equivalent efficacy than CAR-T to which the existing FMC63 was applied. On the other hand, by using a novel epitope, differentiation in the mechanism of action is also expected.
There are four major unmet needs that Abclon is keeping an eye on. First, CAR-T therapy showed an unprecedentedly high complete response rate (CR) in lymphoma patients, but there are still patients who relapse or do not respond to treatment. In diffuse large B-cell lymphoma (DLBCL) as a major indication for Kymriah, the rate of patients not progressing or dying (PFS) 5 years after CAR-T administration was 31%, and that rate in follicular lymphoma (FL) with a relatively better prognosis. was 43% (doi: 10.1056/NEJMc2030164).
Second, after analyzing the malignant B cell sequence of a patient who received CAR-T (FCM63) and relapsed, there was also a study result that CD19 mutation occurred and malignant CD19+ B cells could not be removed (doi: 10.1136/jitc- 2020-001150). It is also known that 10-20% of patients are initially refractory to FMC63 (doi: 10.1038/s41571-019-0184-6). New treatment alternatives are needed for patients who are refractory from the beginning or patients who have relapsed after drug administration. In order to overcome these limitations, CAR-Ts targeting CD20 and CD22 are being developed globally, but their efficacy and target range are relatively limited compared to CD19 targets.
Third, it is expected to improve immunogenicity and durability. According to a previous study, a clinical trial with a CD19 CAR-T to which a humanized scFv of a rodent FMC63 sequence was applied, it was reported that the durability in the body was prolonged and it was likely to lead to higher efficacy (doi: 10.3389/ fimmu.2020.581116). “We are proving the differentiation of AT101 through clinical trials,” said CEO Lee. “So far, there have been no noticeable results in the new CD19 CAR-T clinical trial with an epitope differentiation strategy in the global market,” he added.
Lastly, Kymriah is the only CAR-T treatment that has received marketing approval in Korea, and only third-line DLBCL treatment or B-cell acute lymphocytic leukemia (ALL) patients are covered by insurance. DLBCL (~51%) is the type that accounts for half of all B-cell NHL; rest of the patients with other types such as FL (~27%), mantle cell lymphoma (MCL, ~6%), and marginal zone lymphoma (MZL, 16%) do not receive treatment benefits.
In May of last year, Abclon started phase 1 clinical trials in Korea targeting 82 patients with relapsed or refractory B-cell non-Hodgkin lymphoma (B-cell NHL) by administering AT101 in a tertiary treatment setting to evaluate it. The first administration was provided by professor Duk-Hyeon Yoon at the hospital's Hemato-Oncology Department (NCT05338931). The announcement of the first interim results of the AT101 phase 1 clinical trial was conducted by Professor Marco Ruella of UPenn, who is conducting a joint research, and data showing the differentiation of AT101 compared to Kymriah was released.
An open-label, non-randomized, multicenter phase 1 study was conducted at three different dose levels in patients with relapsed / refractory B-NHL. The results of the first two dose levels in Phase 1, low and medium, have been determined. For medium dose, although the dosage administered to patients is lower than that of currently available CAR-T therapies, all three patients achieved complete response (CR) four weeks after administration.
Even at 5-fold lower dosage to medium dose, CR was observed in 3 out of 6 patients and partial response (PR) was observed in 2 patients. Cytokine release syndrome (CRS) and neurotoxicity (ICANS), which appear as side effects of CAR-T therapy, were also observed at low levels of 11.1% and 22.2%. In particular, the rate of 3 or higher grade on side effects was 11.1%, showing encouraging signs with regard to safety. Clinical trial of high dosage level is currently in progress.
AT101 was administered to patients with diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL). Effective responses to AT101 potentiate the application of AT101 for different types of hematologic malignancies.
Abclon has its own 1140L scale CAR-T GMP production facility for clinical trials, and can produce about 100 samples per year, so it is judged that it can sufficiently cover up to phase 1/2 clinical trials. In addition, the failure rate of clinical samples was minimized by applying an automated system. Abclon was selected as a non-clinical and phase 1/2 clinical support project from the Korea Drug Development Fund (KDDF) twice, respectively, and through the previous project selection, a wide range of non-clinical study was conducted for safety issues such as off-target and toxicity profile.
Regarding future plans, CEO Lee said, “While conducting phase 2 clinical trials of AT101 in Korea, we are thinking to find overseas partners or jointly enter the market through the announcement of clinical results. After analyzing the market, I think it's possible to commercialize and manufacture it by ourselves, so I'm considering several options."
Still, CEO Lee smiled confidently, saying, “Despite the recent difficult situation in the pharmaceutical and bio industries at home and abroad, I feel great pride and responsibility that Abclon’s self-developed CAR-T entered the clinical development stage and saved patients.”

Scalability③ – Solid cancer ‘switchable CAR-T’
If the previous AT101 program is an attempt to prove Abclon’s antibody technology in CAR-T, the switchable ‘zCAR-T’ technology with an on-off concept is a project that shows a long-term vision. Since 8 years ago, Abclon started a joint research on next-generation CAR-T with Seoul National University Professor Joon-ho Jung's team, and has continued to develop it.
zCAR-T is inactivated or low activity, "off", state when administered as it is, but it becomes activated, ‘on’, state when a switch molecule that can activate it is administered. At this time, the core switch molecule is a form in which cotinin and affibody are combined. CAR-T recognizes cotinin while affibody recognizes a cancer antigen. It is expected that the degree of activity can be adjusted based on the concentration of switch molecules administered. Cotinin is a nicotine metabolite that is originally not present in the body and is known to be safe even at high concentrations.
CEO Lee said, “zCAR-T is a challenging task targeting solid cancers. It is difficult to manufacture new CAR-Ts every time because the cancer antigens to be targeted are different depending on the type of solid cancer or patient. Therefore, it is a strategy to manufacture CAR-T first and then add necessary switch molecules according to the patient. Relatively, advantages are expected in terms of CAR-T’s on-off status, active intensity control, overcoming drug resistance, and combined administration. From the standpoint of the manufacturer, it is only necessary to produce one form of CAR-T.”
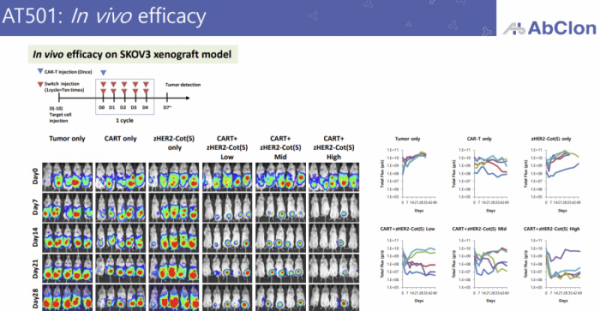
The lead program to which zCAR-T technology is applied is the HER2 target, ‘AT501’. Currently, it is in the final stages of non-clinical trials, and additional improvements are currently being made to improve its efficacy. AT501 is primarily targeting HER2-positive ovarian cancer which has limited treatment options.