기사본문
Genemedicine's 'GM-oAd' Strategy to Overcome Anti-Cancer Virus ‘Limitations’?
입력 2021-06-21 09:25 수정 2021-06-21 09:25
by Shinyoung Roh

GeneMedicine is a bio-company specializing in anti-cancer adenovirus. It was founded in 2014 by Professor Chae-ok Yoon of the Department of Biotechnology, Hanyang University. Since its establishment, GeneMedicine has achieved the outcomes of signing 12 technology transfer contracts with companies at home and abroad in addition to two license-outs with U.S. bio-companies in 2016. In May of this year, GeneMedicine is attracting about 30 billion won of investment through Series B funding.
For the past 25 years, CEO Yoon has been focusing on anti-cancer virus research at Harvard Medical School, College of Medicine of Yonsei University, and College of Engineering of Hanyang University. Therefore, she could clearly see the various limitations of the anti-cancer virus.
“The biggest difference with GeneMedicine is that, since we have studied only anti-cancer viruses for a long time, we precisely understand the limitations of anti-cancer viruses, and we find ways to overcome them during development.” said CEO Yoon.
If so, what are the limitations of anti-cancer viruses that CEO Yoon talked about? And how did GeneMedicine try to overcome them?
◆ ‘Unmet needs’ of anti-cancer viruses, what are the limitations to overcome?
First, though they are anti-cancer viruses, the low specificity for cancer cells can lead to a decrease in the overall efficacy of anti-cancer virus and result in side effects. The low specificity for cancer cells, such as cancer cells gaining resistance to anti-cancer viruses via mutations and anti-cancer viruses infecting and killing normal cells, is a problem in developing therapeutic agents.
Second, it is difficult for anti-cancer viruses to penetrate into tumors. The extracellular matrix, which makes cancer cells attach to surrounding tissues or aggregate, becomes thicker and harder as tumors become malignant. Even if anti-cancer viruses infect cancer cells, the effect of anti-cancer viruses may be limited as they cannot penetrate the extracellular matrix, failing to infect surrounding cancer cells.
Third, for metastatic cancer cells, systemic administration is performed to deliver anti-cancer viruses through the bloodstream. In this case, the anti-cancer viruses in the bloodstream are easily cleared through immune response. Accordingly, the number of anti-cancer viruses actually reaching the cancer tissue are limited, the anti-cancer efficacy is reduced, and the administered viruses cause liver toxicity.
Fourth, as proper treatment is not achieved due to the low anti-cancer efficacy of anti-cancer viruses, there is a possibility of cancer cell metastasis or recurrence. If the anti-cancer virus fails to achieve complete cancer cell death and does not show a proper therapeutic effect, the risk of cancer recurrence and cancer cell metastasis increases.
How does GeneMedicine overcome these limitations of anti-cancer viruses?
◆ ‘GM-oAd’ of GeneMedicine, the answer is ‘genetic engineering
CEO Yoon explained that for GeneMedicine's anti-cancer adenovirus, the shortcomings of the anti-cancer virus were supplemented and its advantages were increased through genetic engineering. In addition, since cancer cell growth mechanisms are various, simultaneous targeting of multiple oncogenic mechanisms by expressing multiple recombinant genes can increase the anti-cancer efficacy and reduce the resistance and recurrence rate of cancer.
CEO Yoon emphasized the followings. GeneMedicine's anti-cancer adenovirus 'GM-oAd' ▲proliferates actively only in cancer cells, ▲exhibits selective anti-cancer effect due to its high cancer cell specificity, ▲does not proliferate in normal cells, thereby being destroyed naturally, and ▲ has a high expression rate of recombinant gene compared to its competing anti-cancer viruses.
In particular, CEO Yoon said, “Compared to competing anti-cancer adenoviruses, the expression rate of the inserted therapeutic gene is high. The 'proliferation capacity' of the virus is also related to the gene expression level of the anti-cancer virus and the resulting cancer cell-killing efficacy." The higher the replication capacity of the virus, the higher the anti-cancer efficacy according to the gene expression of the anti-cancer virus.
The proliferation capacity and gene expression level of anti-cancer adenovirus are regulated through genetic engineering of regions such as promoter, enhancer, and silencer.
How can anti-cancer viruses overcome the problem of extracellular matrix? GeneMedicine explains that the propagation of viruses between cancer cells can be increased by inhibiting the formation of extracellular matrix and removing collagen from the extracellular matrix layer through the insertion of 'decorin' or 'relaxin' gene to the anti-cancer adenovirus. The inserted Wnt inhibitor induces apoptosis by inhibiting Wnt signaling. In addition, it inhibits metastasis and recurrence of cancer. As a result, GM-oAd with the two genes inserted can pass through the extracellular matrix and selectively kill cancer cells, thereby exerting anti-cancer effects.
Optimization of anti-cancer viruses in the tumor microenvironment was also considered. CEO Yoon said, “We have verified that the viral proliferation is also affected by the hypoxic condition of tumor microenvironment where anti-cancer viruses act. To overcome this, we have constructed anti-cancer virus systems.” (doi: :10.1038/s41598-018-20268-6).
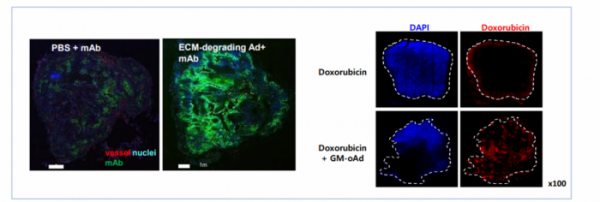
▲'GM-oAd' break down the extracellular matrix and induce spreading of viruses throughout tumor sites (Genemedicine)
◆ A lead candidate ‘GM101’ and a new candidate ‘GM104’
'GM101' is a lead candidate developed by GeneMedicine for the purpose of treating solid cancer. CEO Yoon explained that the cancer cell specificity and cytotoxicity of GM101 as an anti-cancer virus were increased through genetic modification. Containing genes that can break down the extracellular matrix, it can induce spreading of viruses throughout tumor sites, being expected to have a high anti-cancer effect.
GeneMedicine confirmed tumor growth inhibitory effect, extracellular matrix reduction effect, and spreading of anti-cancer viruses in tumors using models for brain tumor, glioma, cervical cancer, and liver cancer through preclinical studies.
GM101 exhibited anti-cancer effect in patients with recurrent cancer. GeneMedicine confirmed that the size of melanoma was reduced by 80% as a result of GM101 injection in patients with malignant melanoma in a phase 1 clinical study. In patients with recurrent lung cancer and liver cancer, the size of lesion was reduced following GM101 injection, and systemic treatment effect was also found after local injection.
CEO Yoon said, “The phase 1 clinical study of GM101 has been completed now. The safety and effectiveness of its intratumoral administration were confirmed.” and “We are planning to start a phase 2 clinical study with GM101 next year.”
In addition, GeneMedicine recently added a new candidate substance 'GM104' to its pipeline. GM104 was designed as an anti-cancer virus for 'cold tumor' in an immunosuppressed state showing no significant immune response to treatments. Through preclinical studies, combinational administration of GM104 and immunological anti-cancer drug was confirmed to have higher anti-cancer efficacy than single administration of immunological anti-cancer drug or GM104. It also showed the possibility of converting tumors into 'hot tumors' having sensitivity to anti-cancer drugs by promoting the spreading of immunological anti-cancer drugs in the tumor microenvironment.
In addition, Gene Medicine has two more candidates. 'GM102' is a pancreatic cancer-targeting anti-cancer virus that contains a recombinant gene for 'decorin + Wnt inhibitor', especially to overcome the intratumoral microenvironment of pancreatic cancer. Currently, non-clinical study of GM102 is under way.
Candidate 'GM103' is an anti-cancer virus under development targeting indications for metastatic lung cancer and liver cancer. In particular, CEO Yoon explained that the two candidates, GM103 and GM104, have high activity to convert 'cold tumors' showing no significant response to treatments into 'hot tumors'. It is because GM103 can convert a cold tumor into a 'hot tumor' by enhancing the penetration of T cells into the environment surrounding the 'cold tumor' that do not have immune cells concentrated in the tumor tissue.
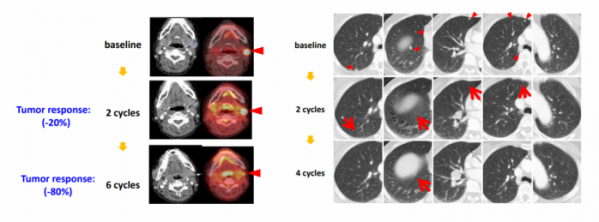
▲cancer cell specificity and cytotoxicity of GM101 (Genemedicine)
◆ GeneMedicine's systemic administration platform even to metastatic cells in blood vessels
When the cancer progresses to a certain level, “metastasis” occurs, which separates from the tumor site and spreads through blood vessels and lymphatic vessels. Since metastatic cancer is difficult to target by local administration, anti-cancer viruses are delivered through the bloodstream to target cancer cells. In this case, the efficacy of the anti-cancer virus rapidly decreases. It is because the immune system recognizes the anti-cancer virus as an antigen, thereby eliminating the virus through immune response.
GeneMedicine's conjugate of macromolecules and anti-cancer virus is a systemic administration platform for targeting metastatic cancer. To prevent the anti-cancer virus from being recognized as an antigen in the immune system, the viral surface is coated with stable polymers that the immune system cannot recognize. However, in case of coating the virus surface, the cancer cell infection rate is lowered. To overcome this, the anti-cancer virus was improved by attaching a cancer cell-specific target protein for normal targeting of cancer cells.
Once GeneMedicine's platform binds to specific receptors on cancer cells and penetrates into the cancer cell, the coating is disintegrated and the virus proliferates within the cancer cells, thereby exerting anti-cancer effects. This platform is currently being applied to GeneMedicine's candidates GM102 and GM104 to develop anti-cancer virus therapeutics for systemic administration.
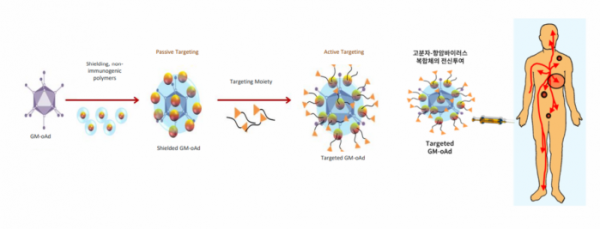
▲Genemedicine's systemic administration platform (GeneMedicine)







