기사본문
Tomocube, Insights From the Customer 'Holotomography 2.0'
입력 2022-07-14 11:02 수정 2022-07-14 11:02
by Sungmin Kim

New imaging techniques can enable the diagnosis or discovery of new drugs that were previously impossible. As a representative example, Eikon Therapeutics attracted a total of $666 million in large-scale investments through Series A and B only last year and early this year. Against this backdrop, Roger Perlmutter, who is the R&D lead of Keytruda at MSD, is the CEO, and the co-founder of the scientist who developed super-resolution microscopy technology and co-won the Nobel Prize in chemistry. This technology has a nanometer (nm) resolution and is recognized for its innovation as it can track each molecule of proteins from living cells. However, the more fundamental reason why Eikon was recognized for its high value is that it built imaging technology in the form of "industrialization" that allows high-throughput experiments.
In that respect, the new attempt of Tomocube draws attention. Tomocube was founded based on an image technology that was never before called holotomography, and brings a new product to the market after 5 years. After a long wait, on the 7th of this month, HT-X1, a new holotomography (Considered to be Holotomography 2.0), was officially launched in the global market including Korea, the United States, and Europe. The concept of holotomography is the same as that it can quantify the three-dimensional image of living cells without label-free, which Tomocube has put forward so far.
However, the meaning of the newly released HT-X1 product is completely different from the previous ones. If the existing product stayed in the research market, this product has now been implemented at a level that can be directly applied to the industry. In other words, biotech, pharmaceutical companies, and large labs now can make products that can easily perform high-content screening experiments on a large-scale.
Since its establishment in 2015, Tomocube has been focusing on making holotomography microscopes and promoting technology. Tomocube released its first holotomography microscope, HT-1S, in 2016, and the following year, the second product, HT-2H, equipped with fluorescent imaging technology. After the product was released, about 200 papers from all over the world have been published using Tomocube's holotomography microscope so far.
However, at a time when products came out one after another and began to be distributed in earnest to the research market, internally, concerns were rather beginning. "I thought we needed new platform technology five years ago," said Yongkeun (Paul) Park, CTO of Tomocube. “As we were demonstrating with existing products, we received feedbacks on the application of noise and multi-well caused by using laser light. However, there was no technology to solve these problems at that time, and a new principle was needed to scientifically implement the technology required by the market. Tomocube and KAIST developed a source technology that we thought was possible, and based on that, we started again for commercialization after more than three years of research.”
Tomocube decided to apply an LED-based optical system instead of a laser to holotomography. It took a considerable amount of time as it was not made with reference to competitor's products or applied existing technology to meet the needs of the market. When the optical system was ready, the internal team led by team leader Sumin Lee began to move quickly.
Tomocube approached it backwards. Instead of starting with a technology developer and developing a product, it rather focused on what functions the market wanted thoroughly, and then all the process was done according to the needs of the users. The HT-X1 product development team collected feedback and information received during the sales process, and began internal planning by collecting opinions from domestic and foreign research laboratories, pharmaceutical companies, hospitals, and imaging device companies, etc. in one place.
In this process, the principle that Tomocube insisted was not to try to change the customer, but to adapt to the customer even if it took time. After the release of holotomography, feedback from almost all users was a request to make applicable a multi-well plate that can take multiple experimental conditions at the same time in addition to a confocal dish that can measure only one image (condition).
Nanolive, Tomocube's only 3D holographic competitor, also identified and moved this need earlier. Nanolive also had a laser-based holographic microscope portfolio and had the same problem. Nanolive released its own customized 96-well plate to solve the problem of multi-well. However, since the thickness is adjusted according to the microscope, the volume of the manufactured well is 100 μl, which is 3 to 4 times smaller than before.
Tomocube did not compromise with reality. Team leader Sumin Lee said, “Since biological experiments are sensitive to quality control and protocols, researchers do not use multi-well if its materials changed. In addition, if customized multi-well, expandability may be limited.” “Our R&D team decided that it was the right way to let customers use what they used, and we tried to solve this problem somehow.”
The result of this process is the world's first LED-based holotomography microscope. The name HT-X1 was given to mean that it is a new product line rather than an improvement of the existing form.
There are three parts that HT-X1 has changed compared to existing products.
First, a commercialized multi-well image plate may be applied. Applicable from 50mm imaging dish to 6-well (35mm), 12-well, and up to 24-well. Biotech or pharmaceutical companies can simultaneously measure various experimental conditions, such as testing the efficacy of drugs/control groups in cancer cell lines by concentration.
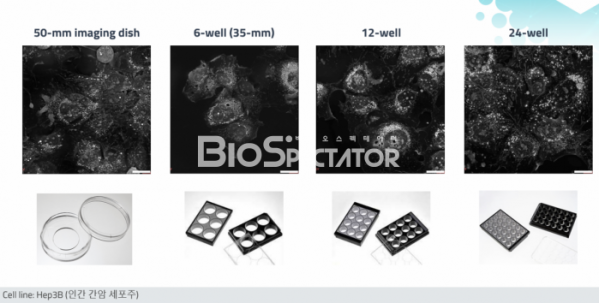
Second, image reproducibility is improved. In the case of the existing HT-1S and HT-2H series, background calibration was required due to image noise generated by using a very sensitive laser, and sensitive adjustments such as distance adjustment were required. Therefore, you have to be very skilled to get a good image. On the other hand, in the case of the HT-X1, the noise problem is solved, and it is equipped with an autofocus function, so both beginners and experts can get the same image by pressing a button. In addition, as the noise disappears, organelles such as mitochondria, nuclei, and lipid droplets can be observed more clearly at high resolution (150 nm).
Third, the applicable sample width has become larger. Existing holotomography products made it difficult to observe densely growing cell lines, primary culture, tissues, and organoids due to noise. Since HT-X1 is designed to observe this thicker sword, up to a thickness of approximately 150 μm can be observed.
Team leader Lee said, "We have made stable equipment that integrates an incubator (STX, Tokai HIT) that cultivates cells in the equipment. We have confirmed the same performance as the CO2 incubator in terms of cell proliferation and viability, and it can be measured for up to 72 hours. In addition to these characteristics, the holotomographic image itself is not photo-toxic and can measure living things."
The keyword that Tomocube targets with HT-X1 is ‘imaging of living cells’. Specifically, it is expected to be applied to three industrial fields: ▲Stem cells/immune cells ▲Organoids ▲The direction of extracting various information related to cells in high-resolution during the development of new drugs.
Other specific phenomena include liquid-liquid phase separation (LLPS) such as stress granules, mitochondrial dynamics, lipid droplets, cell-cell interaction, cytotoxicity assay, etc. It is suitable for measuring cell dynamics, bacteria, and nanomaterial delivery.
Tomocube focuses on areas such as blood cell diagnosis, infectious bacteria identification and antibiotic susceptibility, and companion diagnosis based on H&E slides, which have been previously conducted with HT-2H. At the same time, through the release of HT-X1, the organoid-related pipeline is also expected to release.
“With this global launch, we plan to distribute through our current partners in the US and Europe. Already six months ago, it was purchased from ETH Zurich in Switzerland and is being used” said Sungho Ko, COO, “In addition, we are discussing with two global companies, and a meeting is being held with inquiries from multinational pharmaceutical companies/biotech companies. We will be able to disclose the results when the results come out.”
Kihyun Hong, CEO of Tomocube, said, “With the launch of this high-throughput holotomography product, we expect that Tomocube will be an important turning point in growing from a small startup to a global company.”